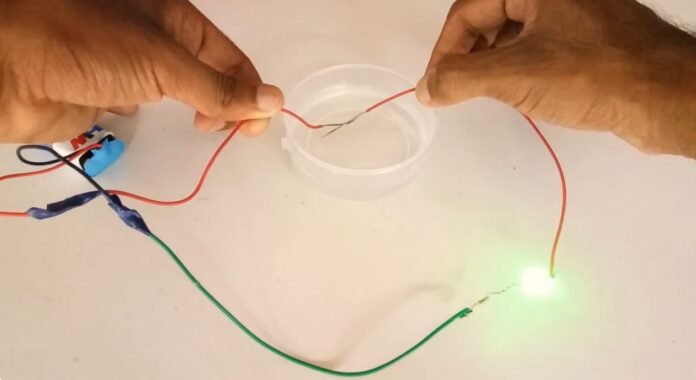
Introduction
We know that many substances conduct electricity through them such as metals, graphite, semiconductors, etc. But there are other substances that do not allow current to flow through them such as wood, plastics, etc. The elements which allow current to flow through them are called conductors and those that do not allow the flow of electricity through them are called insulators. So if the material is allowing the flow of current then we can say that the conductivity of that material is high. And if a certain material does not allow the flow of current then we can conclude that the material in consideration has very low conductivity. It is important then to define what is conductivity.
Electrical conductivity is a measure of the ability of a substance to allow the electric current to flow through it. Metals are having high conductivity among all solids. Carbon is the only gas when solidified as graphite can conduct electricity. On the other liquids can conduct electricity by other means. Unlike metals, the chemical bonding in liquids does not allow electrons to move freely. This means we need to provide a charge into the liquid before it starts conducting.
Do liquids conduct electricity?
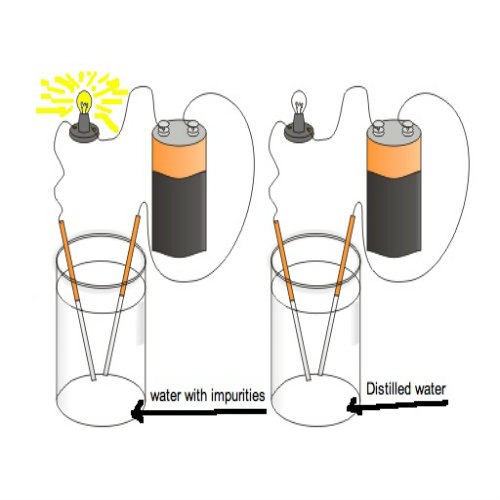
If you take pure water or distilled water then that water is a poor conductor of electricity and it doesn’t allow current to flow through it. But if you add a little bit of impurity to it then it becomes a very good conductor of electricity and it will allow current to flow through it easily. Now what type of impurities can be added to water to make it a good conductor? The answer is salt (NaCl), base (NaOH), or acidic (HCl, H2SO4).
The conductivity of electricity in acidic solutions:
When acids such as HCl, H2SO4, or any other acid are added to water it forms an acidic solution. When two electrodes connected to positive and negative terminals of a battery are dipped in this acidic solution then electrolysis of the solution takes place which creates positively charged H+ ions and negatively charged Cl– ions. Then the positively charged ions start moving toward the Negative electrode and negatively charged ions start to move toward the positive electrode. This establishes a continuous flow of charges through the circuit which gives rise to a sufficient amount of current which is then turned on by the lamp connected to the circuit.
The conductivity of electricity in base solutions:
When bases such as sodium hydroxide (NaOH) or potassium hydroxide (KOH) are added to water then it forms a base solution. When two electrodes connected to positive and negative terminals of a battery are dipped in this acidic solution then electrolysis of the solution takes place which creates negatively charged OH– ions and positively charged, Na+ or K+ ions. The positively charged ions start moving toward the Negative electrode and negatively charged ions start to move toward the positive electrode. This establishes a continuous flow of charges through the circuit which gives rise to a sufficient amount of current which then turns on the lamp connected to the circuit. ![]()
The conductivity of electricity in Salt solutions:
When a common salt i.e.NaCl is added to water, it forms a saline solution. When two electrodes connected to positive and negative terminals of a battery are dipped in this acidic solution then electrolysis of the solution takes place which creates negatively charged Cl– ions and positively charged Na+ ions. The positively charged ions start moving toward the Negative electrode and negatively charged ions start to move toward the positive electrode. This establishes a continuous flow of charges through the circuit which gives rise to a sufficient amount of current which then turns on the lamp connected to the circuit. ![]()
Observation:
When electricity flows through solids no chemical changes take place but when electricity flows through liquids chemical changes take place. For example, when current flows through a copper wire no chemical changes occur but when the current flows through a copper sulfate solution then electrolysis of the solution takes place and positive copper ions and negative sulfate ions.
From the above discussion, we can conclude that
- Some liquids conduct electric current or (electricity) through them.
- The liquids that conduct electricity are called as conducting liquids.
- When electricity is passed through conducting liquids then chemical changes take place.
- The chemical changes that take place in conducting liquids when electricity is passed through them are called chemical effects of current.
Recommended Articles:
Concave And Convex Mirrors Spherical Mirrors
Concave Convex Lenses: Introduction, Difference, And Applications
Concave Mirror And Convex Mirror: Introduction, Types, Difference, And Applications
Concave lens: Introduction, Terminology, Image, And Application
Concentration of Ore: Introduction, Types and Properties of Ore
No. Pure water or distilled water is a poor conductor of electricity. Yes. Acidic solutions are very good conductors of electricity. Hence they can conduct electricity. There are no free electrons in liquids but when electricity is passed through them ions are created which will act as charge carriers and conduct electricity. Liquids conduct electricity because of chemical effect of electric current which creates charge-carrying ions in the liquid solution. Conduction of Electricity in liquids FAQs
Is pure water a good conductor of electricity?
Can current flow through acidic solutions?
Liquids conduct electricity because of electrons or ions?
Which effect of electric current is responsible for conduction in liquids?