A scientist known as Neil Bohr put forward the hypothesis of the angular momentum of an electron after the failure of Rutherford’s atomic model. If we follow Bohr’s atomic model, we can study the different arrangements of electrons that are located among different orbits around the nucleus. In 1924, De Broglie explained that the angular momentum of an electron is quantized.
In this article, we discuss the Angular Momentum of Electrons in detail along with the De Broglie equation.
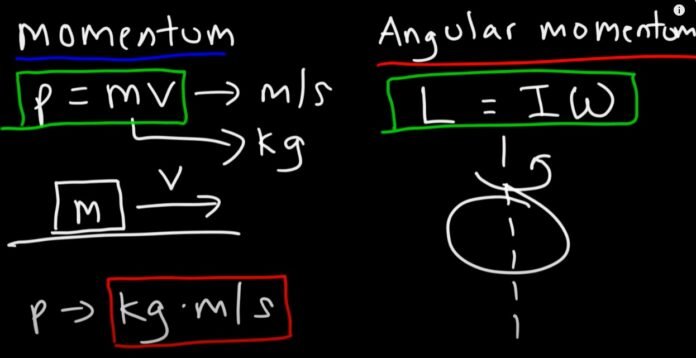
What is the angular momentum of an electron?
After the failure of various Atomic models like Thomson’s Atomic model and Rutherford’s Atomic model, Bohr’s Atomic model was introduced. Bohr’s atomic model laid down various postulates regarding the arrangement of electrons in different orbits around the nucleus. He stated that electrons revolve around the positively charged nucleus in a definite circular path called orbits or shells in an atom and the angular momentum of electrons orbiting around the nucleus is quantized. He further added that electrons move only in those orbits around the nucleus where the angular momentum of an electron is an integral multiple of h/2∏. This postulate regarding the quantization of the angular momentum of an electron was later explained by Louis de Broglie in 1924. Bohr simply postulated certain behavior of atoms without explanation while De Broglie gave a reason for the postulated behavior. According to him, a moving electron behaves like a particle wave in its circular orbit.
The angular momentum of an electron by Neils Bohr is given by mvr or nh/2π (where v is the velocity, m is the mass of the electron, n is the orbit in which the electron is revolving, r is the radius of the nth orbit and h is the Planck’s constant having a value of 6.62607015 × 10-34 m2 kg / s.
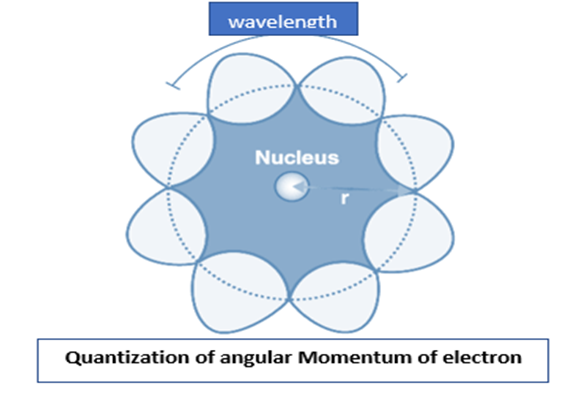
Quantization of Angular Momentum of electron and De Broglie’s Explanation
According to De Broglie, each particle of matter with linear momentum is also a wave. According to him, the electron that revolves around the nucleus in an orbit exhibit both particle and wave character. He further explained why angular momentum might be quantized in the way Bohr imagined it was. He observed that if we take the electron’s wavelength and assume that an integral number of wavelengths must fit in the perimeter of an orbit, we get the same quantized angular momenta as Bohr. According to him, an electron is neither literally a traditional wave nor a traditional particle but is instead a quantized fluctuating probability wavefunction.
The formula of Angular Momentum
For any electron moving in kth circular orbit of radius rk around the nucleus, the total distance is equal to the circumference of the orbit, 2πrk.
| 2πrk = kλ |
|---|
| Let this be the first equation |
Where
λ is the de Broglie wavelength.
As we know de Broglie wavelength is given by:
| λ = h/p |
|---|
Where,
h = Planck’s constant
p is the electron’s momentum
Hence,
| λ = h/mvk |
|---|
Let this be the second equation
Where mvk is the momentum of an electron revolving in the kth orbit.
Inserting the value of λ from the second equation in the first equation we get,
| 2πrk = kh/mvk
mvkrk = kh/2π |
|---|
Hence, de Broglie’s hypothesis successfully proves Bohr’s second postulate which states the quantization of the angular momentum of the orbiting electron. We can also conclude that the quantized electron orbits and energy states of an electron are due to the wave nature of the electron.
Angular momentum of an electron in a Hydrogen atom
When the angular momentum is quantized, the orbit’s radius and energy are also quantized. According to Neil Bohr, transitions of an electron from one allowed orbit/energy to another created the discrete lines in the hydrogen atom’s spectrum. The Bohr model uses quantization of the energy of the electron but was not based on a theory for that quantization. The quantization of angular momentum followed from Bohr’s allowed orbits hence allowing energy levels for the electron. He also believed that, as Albert Einstein stated, energy for a transition is obtained or released in the form of a photon, and that energy is obtained or released in the form of a photon.
∆E=h×v
Where h is Planck’s constant.
The angular momentum of the electron of a hydrogen atom in the ground energy state is h/2 π
Recommended Articles:
Angular Momentum About Fixed Axis
Angular Displacement: Introduction, Motion, and Formula
Angular Acceleration – Formula, Definition, Application, and Factors
The Angle of Incidence
Anemometer – Types and Practical Applications
No electrons have a wave nature also as it was explained was De Broglie in 1924. According to Neil Bohr's second postulate, angular momentum is an integral multiple of 2πh. So, mvr=n(2πh) According to Bohr’s atomic model, the angular momentum of electrons orbiting around the nucleus in an atom is quantized. He further explained that electrons move only in those orbits where the angular momentum of an electron is an integral multiple of h/2π. But this postulate regarding the quantization of the angular momentum of an electron was later explained by Louis de Broglie in 1924. No, Neil Bohr’s Model could explain it only for the Hydrogen atom because he knew that the proton and electron were fixed and could explain the energy of the electron easily. Another factor is that the interaction is only between the proton and the electron. If another electron comes in between, it's hard to detect the position of the electron and to find the distance between them, so we cannot predict the energy of the electrons. The Bohr model uses quantization of the energy of the electron but was not based on a theory for that quantization. The quantization of angular momentum followed from Bohr's allowed orbits hence allowing energy levels for the electron.Angular Momentum of an electron FAQs
Do electrons have only particle nature?
The angular momentum of an electron in the nth orbit is given by?
Is it true that the Angular momentum of an electron is quantized?
Is this true that Neil Bohr's model was able to justify the quantization of angular momentum?