Introduction
The working of science entirely depends on the molecules available in the specific state of matter. In general, the arrangements of molecules in the case of the solid, liquid, and gaseous states are not the same. In the case of solids, the molecules are present close to each other; in the case of gases, they are separated at a considerable distance.
What is an Energy Band?
If we talk about defining the term energy band, it is the number of atoms that are present near each other in a complete crystal stone and will interact with each other. So the electron’s energy level is due to its level of energy.
The most crucial features of the energy band are most common in that the state of the electrons generally remains the same in all the ranges available. So, the concept of an energy band is quite evident in your mind.
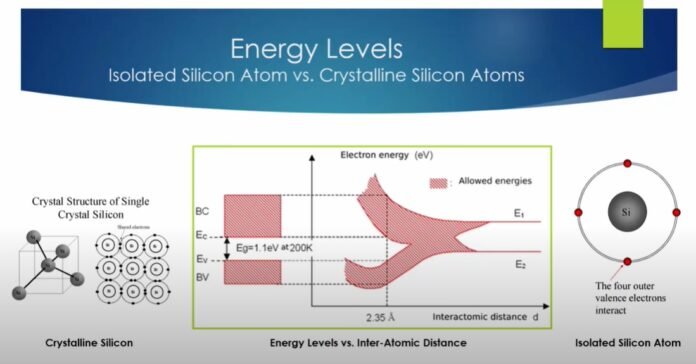
Energy Band Theory
The energy band theory is mainly based on Bohr’s theory. As per the theory, the shells that an atom has contained a different quantity that is available at a dissimilar level. The concept is based on the communication of the electrons available in the inner and outer shells. Let’s look at some basic terms to understand the theory of energy bands better.
- Valence Band
The energy of the electrons in the outer shells is less than the inner shells. The electrons that are available in the outer shell are called valence electrons. The electrons have an energy level that mainly forms the energy band. The band contains the highest level of occupied energy.
Understanding the concept becomes easy if you get complete detail on the terms related to the energy bands.
- Forbidden Energy Gap
The gap that exists between the conduction and the valence band is called the forbidden gap. There is no flow of the electron in the case of this form of the band. If the forbidden gap is more than in that case, the electron in the valence band will be towards the nucleus.
- Conduction Band
The attachment of valence electrons attaches to the nucleus at room temperature. They are called free electrons as they move toward the neighbouring atoms. However, the current flow is there within the conductors of the electrons, so they are called the conduction electrons.
Types of materials based on energy bands
The classification of the materials based on energy bands can be done in mainly three types. All options have features and properties that make them different from other types.
1. Insulators
The best property of the insulators is that electricity cannot flow through them. The crucial examples of insulators are wood and glass. In general, they are materials that have high resistivity. In the case of some of the insulators, the forbidden energy gap is relatively high.
2. Semiconductors
Semiconductors are materials that are known to have less conductivity in comparison to other types of energy bands. The widely used type of Semiconductors in the general market includes Silicon and Germanium. The energy gap between the conduction band and valence band is 1-3eV.
3. Conductors
The availability of the electrons in the case of the conductors is high at room temperature. The most common examples of conductors are Gold and Aluminum. In conductors, the forbidden energy gap is very small or negligible.
Structure of Energy Bands
If we talk about the structure of the energy band, then it is basically the relationship between the momentum of the carrier in solids and the energy. The relationship between the two will vary based on the space in which the electrons are available.
Recommended Articles:
What Is Force Types Of Forces And Their Nature
Internet History of the internet Overview & Timeline
What is Nuclear Fission? Definition, Pros and Cons
PRESBYOPIA—Nearsightedness: Symptoms, Diagnosis, and Treatment
What Is Zero Gravity? Definition, Meaning & Effects
The structure of the energy band is the relation between the carrier's momentum in a solid and the energy. If the electron is in a free space, then the square of the momentum will be proportional to the energy. Mainly the classification of the energy bands is possible in three types: insulators, semiconductors, and conductors. As per the complete research and analysis, there is no real existence of the energy bands. Therefore, without proof, it cannot be concluded that the energy bands exist. The energy bands have mainly been defined based on Bohr's theory. They are mainly the atoms in the particles that are close due to the change in the energy level. Silicon is the best example to define the semiconductor energy bands. What Are Energy Bands? FAQs
What is the basic structure of the energy band?
In how many types is the classification of the energy band possible?
Are there any real existences of energy bands?
What is the concept of energy bands?
What are the crucial examples of semiconductors?