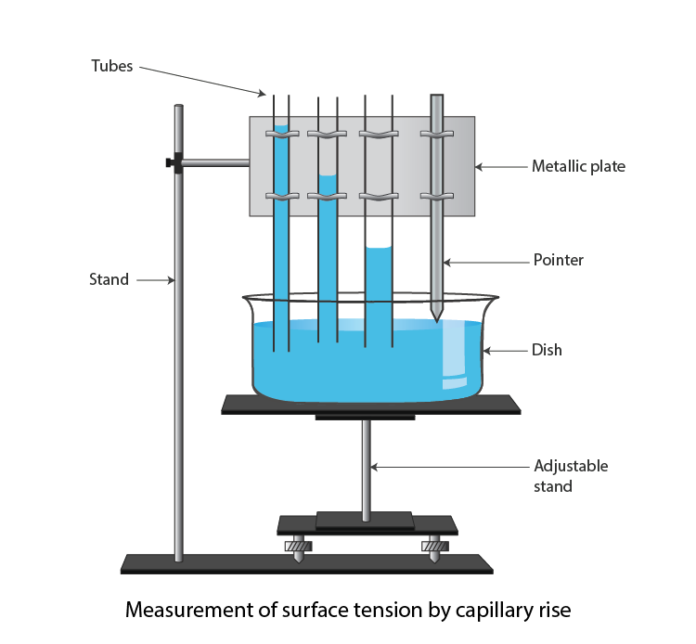
Introduction
Surface tension is a property of a liquid surface due to which it tries to attain the minimum surface area. For example, a small drop of water has a spherical shape because for a given volume the sphere has a minimum surface area. By this property of attaining minimum surface area, the liquid surface tries to attain a stable system.
Aim
To find the surface tension of water using the capillary rise experimental method.
Materials Required for The Experiment
- A glass/plastic capillary tube
- Beaker
- Travelling microscope
- Thermometer
- Dilute nitric acid solution
- Dilute caustic soda solution
- Water
- Plumb line
Principle
When a liquid rises in the capillary tube the surface tension of the liquid is given by:
Where
ρ = density of the liquid,
T= surface tension of the liquid
h = height of the liquid column
r = inner radius of the capillary tube
Procedure
- Clean the beaker and capillary tube with caustic soda and nitric acid and rinse it thoroughly with water.
- Pour water into the beaker and measure its temperature.
- Clamp the capillary tube at one end, above the beaker.
- Move the capillary tube so that the lower end of the tube dips into the water in the beaker.
- Now, push a pin P through a cork C, and fix it just above the water surface. Fix the pin tip such that the tip just touches the surface of the water.
- Now focus the travelling microscope M on the meniscus of the water in capillary A, and move the microscope until the horizontal crosswire is tangential to the lowest point of the meniscus, which is seen inverted in M. Note the reading of the travelling microscope.
- Mark the position of the meniscus on the capillary with a pen. Now carefully remove the capillary tube from the beaker, and then the beaker without disturbing the pin.
- Note the microscope reading by focusing the microscope on the tip of the pin.
- Cut the capillary tube carefully at the point marked on it. Fix the capillary tube horizontally on a stand. Focus the microscope on the transverse cross-section of the tube and take readings to measure the internal diameter of the tube in two mutually perpendicular directions.
Observation/Tabulation
The least count of the microscope = …mm
Determination of h
Measurement of Capillary Rise
| Reading of meniscus h1 (cm) | Reading of tip of pin touching surface of the water h2 (cm) | |||||
|---|---|---|---|---|---|---|
Measurement of the diameter of the capillary tube
| Reading along a diameter (cm) | Diameter | Reading along perpendicular diameter (cm) | Diameter | Mean diameter d | ||
|---|---|---|---|---|---|---|
| One end x1 | Other end x2 | One end
y1 |
Other end y2 | |||
Mean radius r = …cm
Temperature of the water = ….°C
The density of water at 0° C = ….
Calculation
Use the value of h, r, ρ, and g in the formula for T to obtain the surface tension.
Result
The surface tension of water at ….°C = … ± …
Precaution
- Make sure the beaker and the capillary tube are cleaned thoroughly.
- Keep the capillary tube in a vertical position while dipping it in water.
- Make sure the capillary tube is sufficiently wet.
- The water level in the tube should be slightly above the edge.
- Record the temperature before and after the experiment.
- Measure the height of the liquid column from the lowest point of the concave meniscus.
Possible Cause of Error in Experiment
- Inserting dry capillary tube.
- Dirty capillary tube and beaker.
- The capillary tube is not placed vertically while inserted into the beaker.
- Wrong measurement of the microscope reading.
Discussion
- In a capillary tube, the surface of the meniscus is taken to be semi-spherical and the weight of the liquid above the lowest point of the meniscus as .
- Taking the above formula into account the surface tension can be given as . This formula gives a more precise value of the surface tension.
FAQs
Q1. What is the surface tension of water?
Ans. The surface tension of water at 20 °C is .
Q2. What happens if the capillary tube is not wet properly?
Ans. If the capillary tube is not wet properly, the reading obtained will not be accurate. To get an accurate reading, it is important to clean the capillary tube and the beaker properly and to make sure while inserting the capillary tube is wet.
Q3. What is the formula used to find the surface tension by the capillary rise method?
Ans. To find the surface tension of water by capillary rise method the following formula is used. where T is the surface tension of water.
Q4. What is the unit for surface tension?
Ans. Surface tension is given by.
Recommended Articles:
CBSE Class 12th Physics Exam Preparation Tips For Students
CBSE Class 11th Physics Exam Preparation Tips For Students
Finding the Focal Length of a Concave Lens Using a Convex Lens, Steps, Precautions