The behaviour of gas molecules is a topic closely related to the concept of the kinetic theory of gases. The behavior of gas molecules is a key concept in thermodynamics. In order to fully understand the behavior of gas molecules, it is important to first understand the properties of gases and the concepts that describe their behavior.What is gas?
- The gas molecules have no fixed shape or volume and will fill any container to occupy its entire volume. Examples of gases include air, hydrogen, and natural gas.
- It expands indefinitely and uniformly to fill the available space.
- It exerts pressure on its surroundings.
- In gases the intermolecular forces are very weak and its molecules may fly apart in all directions
- They can be easily compressed and expanded, characterized by low density and the ability to occupy a large volume.
- Gas molecules are in constant, random motion and occupy a large volume relative to the volume occupied by individual particles.
What is an ideal gas?
Theoretically, a perfect gas exists. It is thought that a large number of randomly moving point particles make up an ideal gas. Interparticle interactions take place between these particles.
A one-gram molecule of an ideal gas has the same formula as a universal gas constant, absolute temperature, and the number of moles of the gas. The formula for an ideal gas is the product of pressure and volume.
The mathematical formula to determine the ideal gas constant is:
nRT + NkT = PV
Here,
P is the gas’s pressure
V is the gas’s volume
The number of moles is n
R is Universal gas constant
T is the gas’s temperature
N is Avogadro’s number
Assumption of Ideal Gases (or Kinetic Theory of Gases)
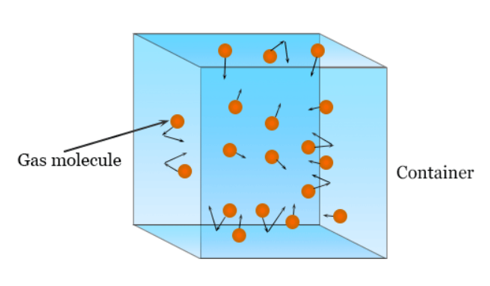
Gas contains a very large number of particles whose sizes are negligible known as molecules. These molecules are in a state of continuous or constant motion or in random motion. During this random motion, molecules continuously move with some velocity and the walls of the container or vessel where it is kept.
This collision is perfectly elastic, almost instantaneous(time duration is small) and between the collision, molecules move in a straight line to acquire uniform velocity.
Also known as kinetic theory of an ideal gas.
Assumption –
● Particles are always continuously moving randomly in all directions but the path it covers is in a straight line.
● Particles are independent of any attractive or repulsive force.
● Gas is collection of large number of tiny particles having small mass like atoms or molecules
● Space between the particles is very large as particles are far away from each other. so compared with the volume of the container, the volume of the particles is negligible.
● Particles are always in constant motion that’s why they have kinetic energy which is directly proportional to temperature
● Due to collision between tiny particles or with containers, collision is perfectly elastic and does not change momentum or energy of the particles. Pressure is also exerted on the container as particles collide with the container. This pressure depends on the number of particles colliding in per unit area.
Factors that affects the behaviour of gas
Temperature – Temperature affects the average kinetic energy of gas molecules, which in turn affects the velocity and frequency of their collisions. An increase in temperature leads to an increase in the velocity and frequency of collisions, resulting in a higher pressure.In simple word, higher temperatures increase the average kinetic energy of gas particles, causing them to move faster and occupy a larger volume.
Pressure – the force exerted by gas particles on the walls of the container is called pressure. It is proportional to the number of particles present, their velocity, and the area of the container. It is affected by several factors, including the temperature, volume, and number of particles present.
Volume: the volume of a gas is directly proportional to the number of particles present, their velocity, and the temperature of the gas.
This means that as the temperature of a gas increases, its volume increases, and as the number of particles in a gas increases, its volume also increases.
Number of particles: the number of particles present in a gas affects its behavior by increasing its pressure and density.
What are the Ideal Gas Laws?
The behavior of gas molecules can be described and predicted using the gas laws. Gas laws are mathematical relationships that describe the behavior of gases under specific conditions. These laws describe the relationships between the pressure, volume, temperature, and number of particles in a gas.These include:
- Boyle’s law
- Charles’s Law
- Gay-Lussac’s Law
- Avogadro’s Law
Boyle’s Law: Boyle’s law states that the pressure of a gas is inversely proportional to its volume, provided that the temperature and number of particles are constant. This means that, if the volume of a gas is increased, its pressure will decrease, and vice versa. This law is often used to describe the behavior of gases in a closed system, such as a balloon or a tire.
Charles’s Law: Charles’s law states that the volume of a gas is directly proportional to its temperature, provided that the pressure and number of particles are constant. This means that if the temperature of a gas is increased, its volume will also increase, and vice versa. This law is useful for understanding the behavior of gases in an open system, such as the atmosphere.
Gay-Lussac’s Law: Gay-Lussac’s law states that the pressure of a gas is directly proportional to its temperature, provided that the volume and number of particles are constant. This means that if the temperature of a gas is increased, its pressure will also increase, and vice versa. This law is useful for understanding the behavior of gases in a closed system, such as a gas cylinder.
Avogadro’s Law: Avogadro’s law states that the volume of a gas is directly proportional to the number of particles present, provided that the pressure and temperature are constant. This means that if the number of particles in a gas is increased, its volume will also increase, and vice versa. This law is useful for understanding the behavior of gases in a closed system.
or
Recommended Articles:
Basic Laws of Physics: Motion, Conservation of Energy, Mass and Thermodynamics
Basic Properties of Electrical Charge
Batteries In Series Parallel
Definition of Beats: Occur, Frequency, Formula, Effect and Applications
Beer-Lambert Law: History, Expression, Derivation and Conditions
Gas is a state of matter where particles are widely spaced, move freely and occupy the entire volume of their container. Gas molecules are in constant, random motion, colliding with each other and with the walls of their container. Gas molecules move in a random, chaotic manner, constantly colliding with each other and the walls of their container. The number of gas molecules in a container affects the pressure they exert on the walls of the container. An increase in the number of gas molecules results in an increase in the frequency of collisions and hence an increase in pressure Intermolecular forces are the attractive or repulsive forces between gas molecules. These forces can affect the behavior of gas molecules, such as the distance between molecules and the frequency of collisions. Behaviour Of Gas Molecules FAQs
What is Gas?
What is the behavior of gas molecules?
How do gas molecules move?
How does the number of gas molecules affect the behavior of gas?
What is the role of intermolecular forces in the behavior of gas molecules?