Introduction
A neutralisation reaction is a chemical reaction that produces salt and water. The pH level after the reaction depends on the strength of the acid and the base. The neutralisation reaction is an exothermic reaction. The process of neutralisation is used to reduce corrosion risks.
To learn more about a neutralisation reaction, keep reading!
What is a Neutralisation Reaction?
In a neutralisation reaction, an acid and a base react to produce salt and water. It doesn’t matter what type of compound an acid and base are, a salt must be able to combine with them. Water always dissolves the salt formed in a neutralization reaction.
Types of Neutralisation Reaction
Acid-base neutralisation:
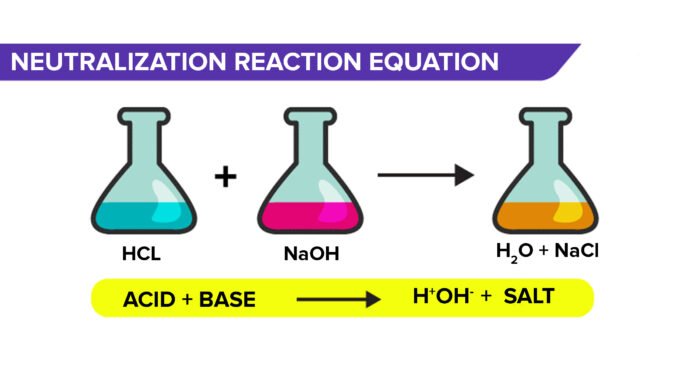
This is a reaction in which an acid and a base combine to form salt and water. For example, sodium chloride (NaCl) and water are formed by a neutralisation reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH).
Precipitation neutralisation:
Acids and bases combine to form precipitates in such a reaction. The precipitate is an insoluble solid that forms as a result of mixing two liquids. For example, when liquid Barium Chloride (BaCl) is combined with liquid Sodium Sulphate (NaSO), the resulting reaction forms a Sodium Chloride (NaCl) solution along with a white precipitate called Barium Sulphate (BaSO).
Oxidation-reduction neutralisation:
In a reaction like this, an acid and a base combine to form an oxidizing agent and a reducing agent. An oxidizing agent is a molecule that accepts electrons from another molecule. A reducing agent is a molecule that donates electrons to another molecule. For example, the reaction between Iron (Fe) and Oxygen (O) creates rust, where the Iron loses electrons while the Oxygen gains electrons.
Neutralisation Reaction Equation
A neutralization reaction is a chemical reaction between an acid and a base. In this reaction, the acid and the base combine to form salt and water, with a pH level of around 7. However, the strength of the acid and the base are instrumental in determining the pH level after the reaction.
The equation for a neutralization reaction is:
Acid + Base → Salt + Water
The equilibrium concentrations of the different species in this reaction are dependent on their respective molalities (molarity).
The concentration of the acid is:
[acid] = (molarity of acid) (litres of acid)
The concentration of the base is:
[base] = (molarity of base) (litres of base)
The concentration of the salt is:
[salt] = (molarity of salt) (litres of salt)
The concentration of the water is:
[water] = (molarity of water) (litres of water)
Neutralization Reaction Application
- A neutralisation reactionoccurs when an acid and a base react to form salt and water. The H+ ion from the acid and the OH– ion from the base combine to form water in this reaction. The salt formed in this reaction is a compound that contains both the positive and negative ions from the acid and base
- The production of baking soda is an application of the neutralisation reaction.Baking soda is created by combining baking soda and an acid. Cream of tartar is the acid used in this reaction. A cream of tartar is a substance containing the tartaric acid molecule. Baking soda and cream of tartar react to form salt and water when combined. This reaction produces sodium tartrate as salt. The white powder, sodium tartrate, is used in baking.
- The neutralisation reactionis also used in treating acidity. The stomach contains acids that aid in the digestion of food. When the amount of acid increases, a person feels a burning sensation. Excess acid is neutralised by using medicines that contain a base, such as an antacid.
Neutralization Examples
- Reaction of sodium hydroxide with sulfuric acid
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
- Reactions of non-metals and bases
2NaOH + CO₂ → Na₂CO₃ + H₂O
Ca(OH)₂ + CO₂ → CaCO₃ + H₂O
- The reaction of metal oxides with acids
CuO + 2HCl → CuCl₂ + H₂O
- Other neutralization reaction examples
H₂CO₃ + 2KOH → K₂CO₃ + 2H₂O
Fe(OH)₃ + 3HNO₃ → Fe(NO₃)₃ + 3H₂O
Ba(OH)₂ + 2HNO₃ → Ba(NO₃)₂ + 2H₂O
3Ca(OH)₂ + 2H₃PO₄ → Ca(PO₄)₂ + 6H₂O
Frequently Asked Questions (FAQs)
Q1: What exactly is a balanced neutralisation equation?
Ans: A chemical reaction involving the mixture of a potent acid and a potent base may be referred to as a neutralisation equation. Typically, the products of such a reaction are water and salt. Because the formula for acid is HCl, the formula for caustic soda is NaOH.
Q2: What is the outcome of the acid-base neutralisation reaction?
Ans: The outcome of acid-based neutralisation is salt and water in a solution. The product may vary when the neutralisation reaction is precipitation neutralisation or oxidation-reduction neutralisation.
Q3: What is a common use of neutralisation reaction?
Ans: Vinegar is often used to treat wasp stings as it neutralises the base in the venom due to its acidic nature. Toothpaste contains ingredients that help neutralise the acid in the mouth as a result of food consumption.
Q4: What is the purpose of neutralisation?
Ans: Farmers use lime to reduce acidic soils (calcium oxide). The stomach contains acid, and plenty of it aids digestion. Antacid tablets contain bases such as milk of magnesia and magnesium carbonate to reduce excess acid.
Q5: How does one neutralise an acid?
Ans: To neutralise acids, a base is used. Bases have a bitter or astringent flavour and a pH greater than 7. Many people use sodium hydroxide, potassium hydroxide, and ammonium hydroxide.