Introduction
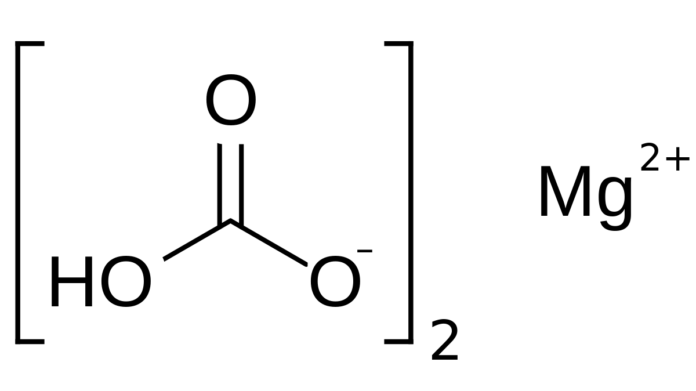
Magnesium bicarbonate is a compound formed by the reaction of magnesium carbonate and carbonic acid. It is a naturally occurring mineral that is found in many sources of drinking water. Magnesium bicarbonate is an important source of magnesium and is used in various applications. In this article, we will explore the properties, uses, and effects of magnesium bicarbonate.
Physical properties of Magnesium Bicarbonate:
1) Solubility: Magnesium bicarbonate is a highly soluble compound that dissolves easily in water. It forms an alkaline solution when dissolved in water due to the bicarbonate ion present in the compound. The solubility of magnesium bicarbonate in water increases with an increase in temperature.
2) Colour and Appearance: Magnesium bicarbonate is a colourless and odourless compound. It is available in the form of a white crystalline powder that can be easily dissolved in water.
3) Density and Melting Point: The density of magnesium bicarbonate is 2.08 g/cm³. It does not have a melting point, as it decomposes at high temperatures to form magnesium oxide, carbon dioxide, and water.
4) Stability: Magnesium bicarbonate is a stable compound that does not decompose easily under normal conditions. However, it can decompose when exposed to heat or strong acids.
5) pH: The pH of a magnesium bicarbonate solution is alkaline due to the bicarbonate ion present in the compound. The pH of a 1% solution of magnesium bicarbonate is around 8.5.
6) Reactivity: Magnesium bicarbonate is not a reactive compound, and it does not react with most organic or inorganic compounds. However, it can react with strong acids, such as hydrochloric acid, to form magnesium chloride and carbon dioxide.
7) Molecular Weight: The molecular weight of magnesium bicarbonate is 162.267 g/mol.
Chemical Properties of Mercuric Chloride
1) Acid-base properties: Magnesium bicarbonate is a weak acid. When it is dissolved in water, it releases hydrogen ions (H+) and bicarbonate ions (HCO3). The hydrogen ions make the solution acidic, while the bicarbonate ions make it weakly alkaline. This property makes it useful in various industries, such as the food industry, where it is used as a food additive.
2) Decomposition: Magnesium bicarbonate can decompose under certain conditions, such as high temperature and pressure. When it is heated, it decomposes into magnesium carbonate, carbon dioxide, and water. This property makes it useful in various industries, such as the cement industry, where it is used as a raw material for the production of cement.
3) Oxidation: Magnesium bicarbonate is a reducing agent. It can react with oxidizing agents to reduce them. For example, when it is reacted with chlorine, it reduces the chlorine to chloride ions. This property makes it useful in various industries, such as the water treatment industry, where it is used to remove chlorine from water.
Applications of Magnesium Bicarbonate
Magnesium bicarbonate has various applications in different fields. Some of the notable applications are:
1) Health and Nutrition: Magnesium is an essential mineral that is required for various physiological processes in the human body. Magnesium bicarbonate is a good source of magnesium, and it can be used as a supplement to prevent magnesium deficiency.
2) Agriculture: Magnesium bicarbonate is used in agriculture as a fertilizer and soil conditioner. Magnesium is an important nutrient for plant growth, and its deficiency can lead to stunted growth and poor yield.
3) Water Treatment: Magnesium bicarbonate is used in water treatment to remove impurities and improve the quality of water. It is used as a coagulant to remove suspended particles and turbidity from water.
4) Industrial Applications: Magnesium bicarbonate is used in various industrial applications. It is used in the production of magnesium oxide, which is used in the production of refractory materials.
5) Food and Beverages: Magnesium bicarbonate can be used as a food additive to improve the nutritional value of food. It can also be used as a preservative to increase the shelf life of food products.
Effects of Magnesium bicarbonate
Magnesium bicarbonate is a mineral-rich compound that can provide various health benefits to the human body. Here are some of the effects of magnesium bicarbonate:
1) Improves digestion: Magnesium bicarbonate can aid in digestion by neutralizing stomach acid and regulating bowel movements. It also helps to reduce inflammation in the gut, which can be beneficial for individuals suffering from digestive issues like IBS.
2) Reduces anxiety and stress: Magnesium bicarbonate has been found to have a calming effect on the nervous system, which can reduce anxiety and stress. Magnesium plays a crucial role in the production of neurotransmitters like serotonin, which is responsible for regulating mood and reducing anxiety.
3) Promotes healthy bones: Magnesium is essential for the development and maintenance of healthy bones. It helps to regulate calcium levels in the body and aids in the absorption of calcium into bones. Magnesium bicarbonate also helps to reduce the risk of osteoporosis, a condition characterized by weak and brittle bones.
4) Regulates blood pressure: Magnesium bicarbonate has been shown to regulate blood pressure by relaxing blood vessels and improving blood flow. Magnesium is also essential for the proper functioning of the heart, which can help to prevent cardiovascular diseases like heart attacks and strokes.
5) Reduces muscle cramps: Magnesium bicarbonate can help to reduce muscle cramps and spasms by regulating the levels of electrolytes in the body. Magnesium plays a crucial role in muscle function and can prevent muscle cramps by relaxing the muscles.
FAQs
Q1. Can magnesium bicarbonate be used to treat high blood pressure?
Ans. Magnesium bicarbonate can help lower blood pressure by relaxing the blood vessels and improving blood flow. However, it should not be used as a primary treatment for high blood pressure.
Q2. How much magnesium bicarbonate should I take?
Ans. The recommended dosage of magnesium bicarbonate varies depending on the individual’s age, sex, and health status. It is recommended to follow the dosage instructions on the product label or consult with a healthcare professional.
Q3. Can magnesium bicarbonate be used as a natural remedy for kidney stones?
Ans. Magnesium bicarbonate can help prevent the formation of kidney stones by reducing the level of calcium in the urine. However, it should not be used as a primary treatment for kidney stones.
Q4. Can magnesium bicarbonate be used to treat constipation?
Ans. Magnesium bicarbonate can help improve digestion by neutralizing stomach acid and reducing inflammation in the gut, which can help relieve constipation.
Q5. Can magnesium bicarbonate be used to treat high blood pressure?
Ans. Magnesium bicarbonate can help lower blood pressure by relaxing the blood vessels and improving blood flow. However, it should not be used as a primary treatment for high blood pressure.