Linear combination of atomic orbitals (LCAO) is a theory in chemistry that explains how atomic orbitals in a molecule combine to form molecular orbitals. This concept is essential in understanding the molecular structure and properties of compounds. In this article, we will discuss the conditions required for LCAO to occur and the process of combining atomic orbitals to form molecular orbitals.
What is LCAO?
LCAO stands for Linear Combination of Atomic Orbitals. This concept is used to explain how atomic orbitals (AOs) in a molecule combine to form molecular orbitals (MOs). These MOs are important for understanding the distribution of electrons in a molecule and the molecule’s properties, such as its shape, reactivity, and bonding.
Conditions for LCAO
For LCAO to occur, certain conditions must be met. These conditions are overlap, similar energy levels, and similar symmetry.
1. Overlap
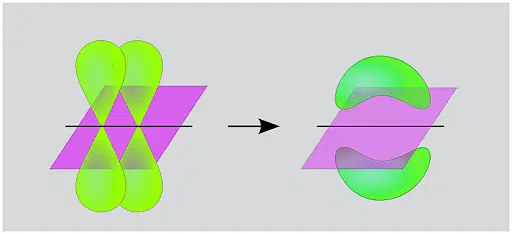
For a bond to form between two atoms, their AOs must overlap. The extent of overlap determines the strength of the bond. When the overlap is maximum, a bond of maximum strength is formed.
2. Similar Energy Levels
The AOs combined must have similar energy levels. The resulting MOs will be intermediate in energy between the original AOs. If AO energy levels are too different, the MOs may be significantly higher or lower in energy than the desired range. In such a case, it would be difficult to make accurate predictions of molecular properties.
3. Similar Symmetry
The AOs combined must have similar symmetry. The resulting MOs inherit the symmetry of the original AOs. Therefore, if the symmetry of the AOs is different, then the MOs formed will have mixed or irregular symmetry, leading to incorrect predictions of molecular properties.
If the above three conditions are met, the AOs can be combined to form MOs, according to the LCAO method.
The Process of LCAO
The LCAO method involves the following steps:
- Step 1: The AOs of individual atoms in a molecule overlap.
- Step 2: The overlapping AOs combine to form new MOs.
- Step 3: The new MOs are filled with electrons in accordance with the Pauli exclusion principle and Hund’s rule.
- Step 4: The resulting distribution of electrons in the MOs determines the molecule’s properties, such as its shape, reactivity, and bonding.
The combination of AOs can occur in different ways, depending on the type of overlap. There are two types of overlap:
- Sigma (σ) overlap: In this type of overlap, the AOs overlap end-to-end along the molecular axis.
- Pi (π) overlap: In this type of overlap, the AOs overlap side-by-side.
Types of Molecular Orbitals
The combination of AOs can result in bonding, antibonding, or non-bonding MOs.
- Bonding MOsare lower in energy than the original AOs. They contribute to the strengthening of the molecule.
- Antibonding MOsare higher in energy then original AOs. They weaken the chemical bond between atoms.
- Non-bonding MOs neither contribute to nor weaken the bond strength.
Advantages of LCAO
LCAO is a powerful concept that allows us to understand the molecular structure and properties of compounds. Some advantages of LCAO are:
- LCAO provides a qualitative understanding of the electronic structure of molecules.
- LCAO can predict the shapes of molecules and their bonding properties.
- LCAO can explain the reactivity and stability of molecules.
- LCAO can be used to design new molecules with desired properties.
Importance of LCAO
The LCAO method is crucial for understanding the nature of chemical bonds. It provides a mathematical approach to describe the bonding of atoms in a molecule. By combining AOs, we can predict the shape and energy levels of the MOs formed. This helps us understand the chemical properties of molecules, such as reactivity, stability, and polarity.
Understanding the conditions required for the Linear Combination of Atomic Orbitals is crucial for comprehending the bonding behaviour of molecules. The proper alignment of orbitals and correct mathematical equations for combining them can result in strong covalent bonds and stable molecules. With this knowledge, we can better understand and predict the chemical properties of substances, opening doors to new discoveries and innovations in the field of chemistry.
Linear Combination Of Atomic Orbitals Conditions FAQs:
Q1. How does the Linear Combination of Atomic Orbitals contribute to the stability of molecules?
Ans. The Linear Combination of Atomic Orbitals (LCAO) method contributes to the stability of molecules by forming new hybrid orbitals that are better suited for bonding. These hybrid orbitals have a lower energy than the original atomic orbitals and are able to form stronger bonds with other atoms. This results in a more stable molecule.
Q2. How does the Linear Combination of Atomic Orbitals explain the molecular geometry of molecules?
Ans. The Linear Combination of Atomic Orbitals (LCAO) method can be used to explain the molecular geometry of molecules by determining the number of hybrid orbitals that are formed and their spatial orientation. The shape of the hybrid orbitals determines the shape of the molecule.
Q3. How does the Linear Combination of Atomic Orbitals differ from the Molecular Orbital theory?
Ans. LCAO is a simple method used to construct MOs by mathematically combining atomic orbitals (AOs) from individual atoms. On the other hand, MO theory is a more sophisticated approach that describes MOs as arising from the wave-like behaviour of electrons in a molecule.
Q4. Can the Linear Combination of Atomic Orbitals be used to explain the properties of solids?
Ans. Yes, the Linear Combination of Atomic Orbitals (LCAO) method can be used to explain the properties of solids. It can describe the bonding between atoms in the solid state. LCAO is commonly used to explain the electrical conductivity and thermal conductivity of metals.
Q5. How does the Linear Combination of Atomic Orbitals relate to the concept of hybridisation?
Ans. Hybridisation refers to the mixing of AOs to form new hybrid orbitals that are better suited for bonding. It can be seen as a special case in LCAO. Hybridisation plays a key role in determining the molecular geometry of molecules.