Introduction
Iridium belongs to the platinum group of metals, is a rare and valuable element found in the Earth’s crust and is also present in meteorites.
This dense, hard, brittle and silvery-white metal has the highest melting point of any element and is highly resistant to corrosion.
Iridium has a wide range of uses in various industries, including aerospace, electronics, and medicine.
Let’s learn its unique properties and its many uses.
Discovery of Iridium
Iridium was discovered in 1803 by English chemist Smithson Tennant.
The name “iridium” is derived from the Greek word “iris,” which means rainbow, because of the various colours that can be seen in its salts.
South Africa is the largest producer followed by Russia and Canada.
Physical properties of Iridium
- Density: Iridium is the second densest element after osmium, with a density of 22.56 g/cm³.
- Melting and boiling point: Iridium has a very high melting point of 2,466 °C (4,471 °F), and a boiling point of 4,428°C (7,982°F).
- Hardness: Iridium is also very hard and brittle, with a Mohs hardness of 6.5.
- Corrosion resistance: Iridium is highly resistant to corrosion and oxidation, making it useful in many applications where these properties are important.
- Electrical conductivity: Iridium is a good electrical conductor, although not as good as copper or silver.
- Radioactivity: Iridium has several radioactive isotopes, but the most common form of iridium is stable and non-radioactive.
- Low coefficient of thermal expansion: Iridium has a very low coefficient of thermal expansion, which makes it useful in high-temperature applications.
- Ductility and malleability: Iridium is not very ductile or malleable, meaning that it is difficult to shape or form into wires or sheets.
- Magnetic properties: Iridium is not magnetic at room temperature, but it becomes weakly magnetic when it is exposed to a magnetic field.
Chemical Properties of Iridium
● Not easily soluble –
Not soluble in most acids, including hydrochloric, nitric, and sulfuric acids. However, it can be dissolved in a mixture of hydrochloric and hydrofluoric acids.
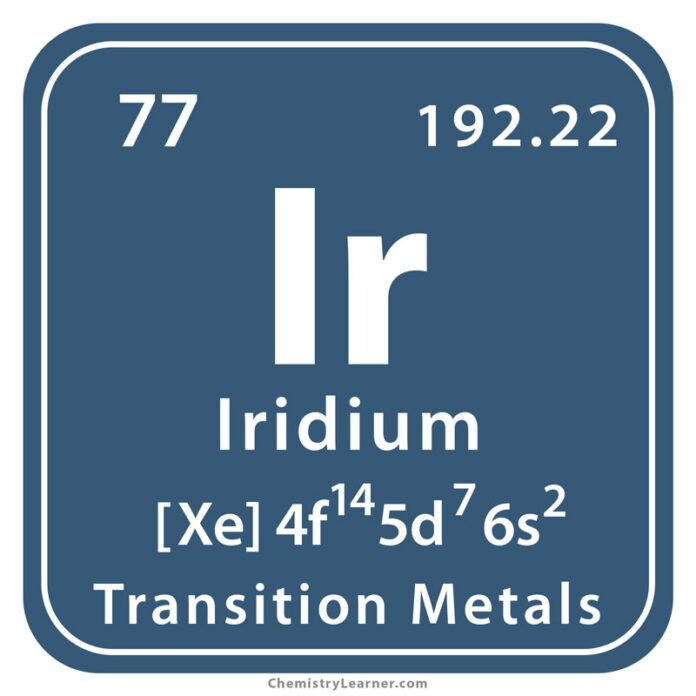
● Chemical symbol – Ir
This is the chemical symbol for iridium, which is used to identify the element in the periodic table.
● Atomic number – 77
This is the number of protons in the nucleus of an iridium atom, which determines its place in the periodic table.
● Electronic configuration –
[Xe] 4f14 5d7 6s2 – This is the arrangement of electrons in the energy levels of an iridium atom, which determines its chemical behaviour.
- Oxidation states– +1, +2, +3, +4, +5, +6 – These are the different ways that iridium can form compounds with other elements, by either losing or gaining electrons.
● Electronegativity –
2.20 (Pauling scale) – This is a measure of the tendency of iridium to attract electrons towards itself when it forms chemical bonds with other elements.
● Ionization energy –
8.967 eV – This is the energy required to remove an electron from an iridium atom, which determines how easily it can form ions.
● Reactivity –
Iridium is a highly unreactive metal and does not react with air, water, or most acids.
This is due to its dense structure and the stability of its electron configuration. This makes it useful in applications where resistance to corrosion and chemical stability are important.
Crystal Structure
Iridium has a face-centred cubic crystal structure with a lattice constant of 3.839 Å.
Its unit cell contains four iridium atoms, which are located at the corners and in the centre of the cube.
Isotopes
Iridium has two stable isotopes, 191Ir and 193Ir, and several radioactive isotopes, including 192Ir, 194Ir, and 195Ir.
Natural occurrence of Iridium
In the case of iridium, a concentration of 0.001 ppm (parts per million) means that for every million parts of the Earth’s crust, there are about 0.001 parts (or 1 milligram) of iridium.
Iridium is often found in association with other platinum group metals, such as platinum, palladium, and osmium, in deposits known as “placers” or “gravel beds”.
It is also found in igneous rocks, such as basalt and gabbro, and in meteorites, where it occurs in high concentrations.
Extraction and production of Iridium
The extraction process involves a series of chemical and physical separation steps, such as froth flotation, gravity separation, and magnetic separation.
Once the iridium-containing ore has been separated from other minerals, it is refined to remove impurities and produce a concentrated iridium compound, such as iridium sponge or iridium powder.
The concentrated iridium compound can then be further processed to produce iridium metal.
Key uses of Iridium
Electronics
Iridium is used in the production of hard disk drives and mobile phones.
Its ability to form a stable and durable layer when used in the production of thin-film coatings makes it an ideal material for these applications.
Automotive Industry
Iridium is used in the production of automobile catalytic converters because of its resistance to high temperatures and oxidation.
Jewellery
Iridium is sometimes used in the production of high-end jewellery due to its rarity and unique properties.
It is often alloyed with other precious metals, such as gold and platinum, to create highly durable and attractive pieces.
Aerospace Industry
Iridium is used in the aerospace industry for various applications, including rocket engine parts, high-temperature coatings, and electrical contacts.
Medical Industry
Iridium isotopes are used in radiation therapy for cancer treatment.
Scientific Research
Iridium is used in various scientific research applications, like X-ray optics and in the study of superconductivity.
Chemical Industry
Iridium is used as a catalyst in the chemical industry, particularly in the production of fertilizers and plastics.
Catalysts
Iridium is used extensively in the production of catalysts for the chemical industry.
These catalysts are used to speed up chemical reactions and improve the efficiency of industrial processes, like the production of fertilizers, plastics, and pharmaceuticals.
Spark plugs
Iridium is commonly used in spark plugs due to its high melting point, durability, and resistance to corrosion.
This makes it an ideal material for use in engines that operate under extreme conditions, such as high-performance race cars.
Methods of Iridium Recycling
Pyrometallurgical – involves the use of high-temperature furnaces to melt and separate iridium-containing materials from other metals and alloys.
Hydrometallurgical – involves the use of chemical solvents to dissolve and separate iridium from other metals and alloys.
Iridium FAQs
Q1. What is iridium, and where is it found?
Ans. Iridium is a chemical element with the atomic number 77 and the symbol Ir. It is a rare, dense, silvery-white metal that is found in the Earth’s crust and is also present in meteorites.
Q2. What are the main uses of iridium?
Ans. It is used in satellite communication systems, spacecraft manufacturing, high-performance capacitors, high-temperature thermocouples, spark plugs, radiation therapy equipment, brachytherapy, platinum alloys in jewellery making, and as a plating material.
Q3. What are the properties of iridium that make it useful in the industry?
Ans. High melting point, high density, excellent resistance to corrosion, and catalytic activity are a few properties.
Q4. How is iridium extracted and produced?
Ans. Iridium is typically extracted as a byproduct of platinum mining. It is also produced through the processing of iridium-containing ores or as a byproduct of other industrial processes such as the production of nitric acid.
Q5. Why is iridium so expensive?
Ans. Iridium is a rare element, with an abundance in the Earth’s crust estimated to be only around 0.001 ppm (parts per million). It is also challenging to extract and refine, often requiring complex and expensive processes.