Introduction
Acids and bases are two of the most important and widely studied substances in chemistry. They have distinct properties that make them useful in many different applications, such as cleaning, food preparation, and scientific research. Understanding the properties of acids and bases is important for different fields, such as medicine and engineering. In this article, we will explore some of the most important experiments on the properties of acids and bases and what they have taught us about these important substances.
The pH Scale
Before we dive into the experiments themselves, it is important to understand the pH scale. The pH scale is a measure of the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral. A solution with a pH below 7 is considered acidic, while a solution with a pH above 7 is considered basic (also called alkaline). The lower the pH, the more acidic the solution. The higher the pH, the more basic the solution.
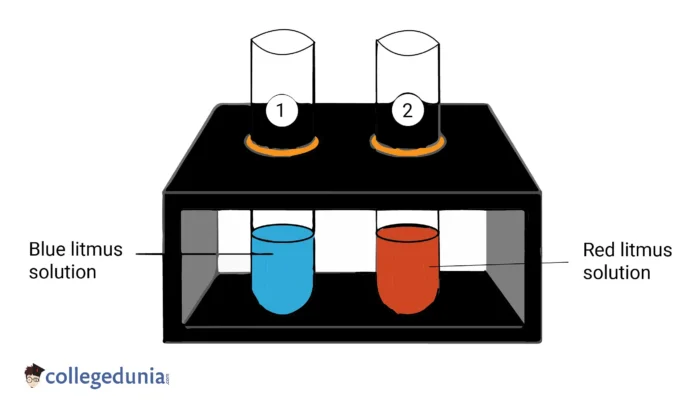
Experiment 1: Litmus Paper
One of the simplest experiments to demonstrate the properties of acids and bases is the use of litmus paper. Litmus paper is a type of pH indicator that changes colour in response to the acidity or basicity of a solution. If litmus paper is dipped in an acidic solution, it will turn red. If it is dipped in a basic solution, it will turn blue. This simple experiment allows us to easily determine whether a solution is acidic or basic.
Experiment 2: Acid-Base Titration
Another commonly used experiment to explore the properties of acids and bases is acid-base titration. In this experiment, a solution of a known concentration (the titrant) is slowly added to a solution of unknown concentration (the analyte) until the two solutions neutralise each other. A common indicator used in acid-base titrations is phenolphthalein, which turns pink in basic solutions and remains colourless in acidic solutions.
By measuring the volume of the titrant needed to neutralise the analyte, we can calculate the unknown concentration of the analyte. This experiment allows us to determine the exact concentration of an acid or base in a solution, which can be important for many different applications.
Experiment 3: Electrolysis
Electrolysis is another experiment that can be used to study the properties of acids and bases. In this experiment, we pass an electric current through a solution containing an acid or base. When an acid is electrolyzed, hydrogen gas is produced at the cathode (negative electrode), while oxygen gas is produced at the anode (positive electrode). When a base is electrolyzed, the same reaction occurs, but hydroxide ions (OH-) are produced at the cathode instead of hydrogen gas.
By measuring the amount of gas produced during electrolysis, we can determine the strength of the acid or base in the solution. Stronger acids and bases produce more gas than weaker acids and bases.
Experiment 4: Reactivity with Metals
The reaction of acids and bases with metals is another important experiment that we can use to study their properties. When an acid reacts with a metal, it produces hydrogen gas. For example, when hydrochloric acid reacts with magnesium, the following reaction occurs:
2HCl + Mg → MgCl2 + H2
When a base reacts with a metal, it does not produce hydrogen gas. Instead, it produces metal hydroxide. For example, when sodium hydroxide reacts with magnesium, the following reaction occurs:
2NaOH + Mg → Mg(OH)2 + 2Na
By observing the reaction between acids, bases and metals, we can learn more about their properties, including their reactivity and strength.
Experiment 5: Neutralisation
The most important experiment on the properties of acids and bases is neutralization.
Neutralization occurs when an acid and a base react with each other to produce a salt and water. For example, when hydrochloric acid reacts with sodium hydroxide, the following reaction occurs:
HCl + NaOH → NaCl + H2O
The products of the reaction are a salt (sodium chloride) and water. The pH of the solution is neutral (pH 7), because the acid and base have neutralized each other.
Neutralization is a fundamental chemical reaction that has many important applications. For example, it is used in the production of soaps and detergents, as well as in the treatment of acidic wastewater.
FAQs
Q1: What is an acid?
Ans: An acid is a substance that, when dissolved in water, increases the concentration of hydrogen ions (H+) in the solution.
Q2: What is a base?
Ans: A base is a substance that, when dissolved in water, increases the concentration of hydroxide ions (OH-) in the solution.
Q3: What is the pH scale?
Ans: The pH scale is a measure of the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral, lower values indicating acidity, and higher values indicating basicity.
Q4: How can I measure the pH of a solution?
Ans: You can measure the pH of a solution using pH paper or a pH meter.
Q5: What are some common acids and their uses?
Ans: Some common acids include hydrochloric acid (used in the production of PVC and in the stomach to aid in digestion), sulfuric acid (used in car batteries and in the production of fertilisers), and acetic acid (found in vinegar).