The enthalpy of neutralization is the change in heat when an acid and a base react to form salt and water. This is usually an exothermic reaction, which means that heat is released. In this article, we will study enthalpy of neutralization of strong acid and strong base.
Introduction:
The enthalpy of neutralization refers to the heat released or absorbed during the acid-base reaction. When a strong acid reacts with a strong base, the resulting enthalpy change is particularly large due to the strength of the reactants. This exothermic reaction leads to the formation of salt and water.
Neutralization Reactions:
In the reaction of acid and base to generate water and salt, the reaction requires the combination of H+ and OH– ions, and this reaction is called neutralization reaction.
exists at pH 7 for the neutralization of heavy acids and bases. In the neutralization of strong acids and strong bases, the neutralized Ph value will be less than 7, and subsequently in the case of strong bases neutralizing weak bases, the Ph value will be greater than 7.
It is also believed that salts are formed by neutralization reactions of equal concentrations by weight of acids and bases, and that N parts of acid will always neutralize N parts of base.
Acid + Base → Salt + H2O
What is Standard Enthalpy Change?
The process of reacting an acidic solution and an alkaline solution to produce 1 mole of water under normal conditions is called the standard enthalpy change. The neutralization shift is measured per mole of water produced in the reaction.
Why Would a Strong Acid Reacting with a Strong Base Produce the Same Value?
Strong acids and bases react to form salts and water in a highly exothermic reaction. The heat of this reaction is called the enthalpy of neutralization. The reason why strong acids and bases react to give similar values is that the reaction between strong acids and bases is highly exothermic and releases a fixed amount of heat energy per mole of water formed.
H+(aq.) + OH– → H2O
In other words, strong acids and strong bases ionize completely in water to form H+ and OH– ions respectively. When they react, these ions combine to form water, a very stable compound with a low enthalpy of formation. Since H+ and OH– ions come from highly stable and fully dissociated sources, the heat released is nearly constant and independent of the properties of strong acids and bases. This results in similar neutralization enthalpy values seen for strong acids and strong bases.
Experimental Methodology:
Aim:
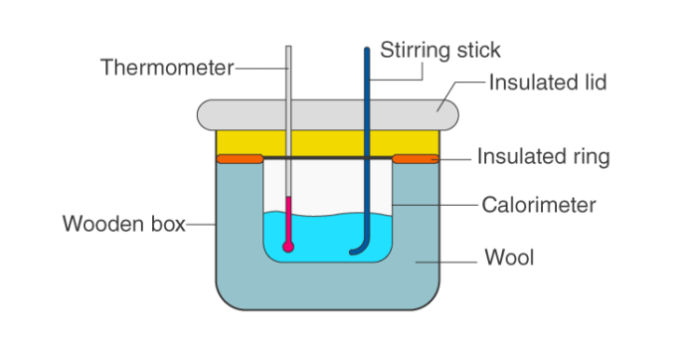
To determine the enthalpy of neutralization of strong acids and bases. The strong acid taken is hydrochloric acid, and the strong base taken is soda.
Theory:
The experiment is run between a strong acid and a strong base using the titration procedure and the resulting temperature at which the reaction goes to equilibrium is recorded as a reading from which the calorific value is calculated.
Also, the enthalpy of neutralization is the heat generated when one gram equivalent of an acid is completely neutralized by a base in dilute solution.
The chemical equation for experiment is:
H+(aq) + Cl–(aq) + Na+(aq) + OH–(aq) → Na+(aq) + Cl–(aq) + H2O + 13.7 kcal
The heat of 13.7 kcal is given off, called the heat of neutralization of all strong acids and bases.
In addition, it obtained a constant value of the heat of neutralization of 13.7 Kcal under almost all strong acids and strong bases. This constant value of the heat of neutralization is explained on the basis of the theory of ions.
Materials Required:
- Sodium hydroxide solution 0.2 M
- Graduated cylinder with contents
- Thermometer for measuring temperature
- Stirrer with cork stopper
- Rubber stopper
- Large polyethylene bottle
- Hydrochloric acid (0.2 M)
Procedure:
- First, calculate the water equivalent of the calorimeter.
- Add an additional 100 ml of 0.2 M hydrochloric acid solution.
- Then read the temperature of the acid solution.
- Next, take a separate container and pour 0.2M sodium hydroxide solution.
- Read the initial temperature of the sodium hydroxide.
- Wait for both temperatures to reach the same temperature.
- After keeping the temperature constant, quickly transfer 100 ml of sodium hydroxide solution into the saline solution.
- Immediately after transfer, adjust the cap.
- After that, stir the solution and take a reading.
- Take frequent readings until the temperature remains constant.
- Correctly take the reading when the temperature is at its highest.
- Calculate the heat generated when two solutions are mixed in the ratio method.
Observations:
- Initial temperature = to1C
- Final temperature = to2C
- Temperature change = (t1– t2)oC
- Mass of mixed solution after neutralization = 200 grams
- Calorimeter water equivalent = W grams
Calculations:
Enthalpy change during neutralization of 100mL 0.2M HCL = (200*W)* (t1 – t2)*4
Enthalpy change during neutralization of 1000 ML 1M HCL = ((200*W)* (t1 – t2)*4.2 / 0.2 )/(1000/100)
Precautions:
- The specific heat of the solution is taken as 4.189J/g.
- They lost very little heat to the environment due to radiation.
- This assumes a solution with a density of 1 g/ml.
- Assume 100% HCL and NaOH.
- All readings should be read carefully.
Frequently Asked Questions – (FAQs):
- How do neutralization reactions take place?
The neutralization reaction occurs when an acid reacts with a base. During the reaction, H+ ions of the acid react with OH– ions of the base to form water (H2O), which neutralizes the acidity of the acid and the base alkalinity. The remaining ions of acids and bases form salts. The resulting solution is neither acidic nor basic, but neutral.
- Can strong acids and weak bases react with each other?
Yes, strong acids and weak bases can react with each other. In such reactions, a strong acid donates a proton to a weak base, forming a conjugate acid and a conjugate base. The resulting solution has a lower pH because the conjugate acid of the weak base is acidic.
- Write the significance of enthalpy of neutralization in real life.
The enthalpy of neutralization gives information about the reaction that occurs between an acid and a base. It also has some real-world applications, for example when a person has acidity, an alkaline solution can be prepared to help relieve the acidity.
- How to judge if the neutralization reaction is exothermic or endothermic?
Exothermic reactions involve the release of heat during the formation of the product, while in the case of endothermic reactions, heat is absorbed. During neutralization, acid and base mix to form salt and water, so neutralization is an exothermic process