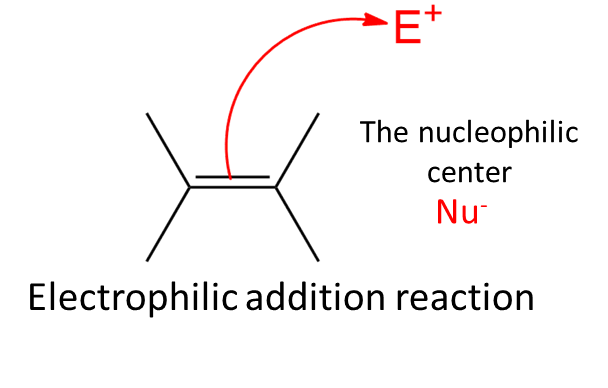
Electrophilic Addition: Numerous organic reactions are well-known to us. Concerning Alkenes, they can experience Addition reactions. In this piece, we will explore further electrophilic addition reactions of alkenes.
Introduction
Addition reactions are a type of chemical reaction in which two or more molecules combine to form a larger molecule. This reaction typically occurs between molecules containing multiple bonds, such as alkenes, alkynes, and dienes. Addition reactions can be classified into several types based on the reactants’ nature, including electrophilic and nucleophilic additions.
What is an Addition Reaction?
The simplest definition of an addition reaction in organic chemistry is a chemical process in which two or more reactants combine to produce a larger single product. However, only chemical compounds with several bond types can undergo an addition reaction since a double or triple bond is typically broken to create the necessary single bonds.
1. Electrophilic addition reactions
Electrophiles are molecules attracted to electrons and tend to react with other molecules by donating a pair of electrons.
- In electrophilic addition reactions, these species combine with another substance to form a product without losing any atoms from the reactants.
- The pi bond between carbon atoms in a double bond is reactive and weak, making it susceptible to functional groups being added to it.
- The pi electrons also increase the electron density on the carbon atom, making it more attractive to electrophiles. Therefore, molecules with double or triple bonds can undergo electrophilic addition reactions.
Mechanism
- The catalyst for this reaction is the production of the electrophile X+, which forms a covalent bond with the electron-rich, unsaturated C=C bond.
- The positive charge on X is transferred to the carbon-carbon bond during the forming of the C-X bond, forming a carbocation.
- At the second stage of electrophilic addition, the intermediate’s generated positive charge joins with an electron-rich species to form the second covalent bond. The reactants and the reaction’s circumstances determine the precise characteristics of the electrophile and the positively charged intermediate.
Electrophilic Addition Reaction of Alkenes
Alkene is subjected to an array of processes, such as oxidation and ozonolysis. Alkenes exhibit electrophilic addition reactions in the following ways:
1. Hydrogenation
Alkenes undergo hydrogenation, a reduction reaction, to add hydrogen. When an organic compound is hydrogenated, it becomes “saturated” with extra hydrogen atoms. As hydrogenation only occurs spontaneously at extremely high temperatures, a catalyst is typically needed throughout the process. The most frequently utilised catalysts are nickel, platinum, or palladium. Hydrogenation occurs when two hydrogen atoms are added to a molecule, usually an alkene.
2. Hydration
A chemical reaction called hydration occurs when two substances interact with water. Alcohol is produced when alkenes react with water in an addition reaction when a catalyst is present. This kind of addition reaction is known as hydration. Direct water infusion occurs within the carbon-carbon double bond. The industrial production of ethanol, isopropanol, and butan-2-ol uses this type of reaction.
3. Halogenation
A halogen-halogen bond becomes polarized when a halogen molecule, such as Br2, approaches the double bond of an alkene. This happens because the double bond’s electrons push the bromine molecule’s electrons away. The halogen-halogen bond subsequently gains a dipole moment. Due to heterolytic bond cleavage, one of the halogens acquires a positive charge and reacts as an electrophile. The alkene picks up the electrophile. This kind of reaction involves both halogenation and electrophilic addition. As a result, a bromonium ion is produced. Moreover, the generated intermediate is attacked by the other halogen, and the halogen subsequently bonds to the alkene.
4. Hydrohalogenation
In the hydrohalogenation of alkenes, the carbon to carbon double bond is broken, and then an electrophilic addition of an atom of hydrogen and a halogen is produced. Markovnikov’s rule states that the halide will increase the amount of substituted carbon. The product of the reaction is a haloalkane, also referred to as an alkyl halide. The carbon from the double bond and the hydrogen from H-Br combine with the two pi electrons to form a C-H sigma bond. After a halide ion is added, a carbocation intermediate is formed, which goes on to produce the end product. This is an example of an electrophilic addition reaction of alkenes since electrophile is added during the process.
Frequently Asked Questions (FAQs)
- What is the primary characteristic of the addition reaction?
An addition reaction occurs when an atom is added to a double or triple bond compound. Unsaturated molecules are related to addition processes. There are no reactant residues left over once an addition reaction is completed.
- Why does an addition reaction occur?
An addition reaction in organic chemistry involves two or more molecules forming a larger one (the adduct). Because they also have double-bond nature, molecules containing carbon—hetero double bonds, such as carbonyl (C=O) or imine (C=N) groups, can be added.
- What exactly is the photo halogenation reaction?
A haloalkane is formed when a halogen combines with an alkane in the presence of ultraviolet radiation or heat. One illustration is the chlorination of methane.
- What do the Markovnikov and Anti-Markovnikov rules entail?
The carbon atom with the greatest number of hydrogen substituents receives the hydrogen atom, according to the Markovnikov rule. By the Anti-Markovnikov rule, the hydrogen atom is attached to the carbon atom with the fewest hydrogen substituents.