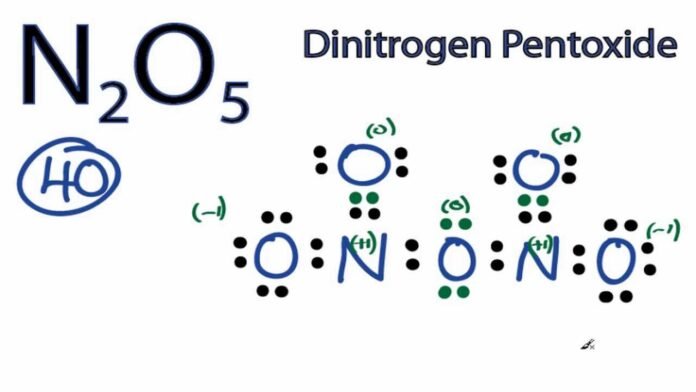
Dinitrogen Pentoxide Introduction:
Dinitrogen pentoxide is a compound which contains nitrogen and oxygen in the ratio of 2:5. One dinitrogen pentoxide molecule has two (di) nitrogen atoms and five (pent) oxygen atoms. The chemical formula of the compound is N2O5.
Nitrogen pentoxide can exist as colorless crystals in the anhydrous form of nitric acid. It melts at 30°C.Nitric pentoxide, a somewhat unstable nitrogen oxide, is used as an oxidizing agent in several chemical reactions. It is a nitriding agent. However, it is now mostly replaced by nitronium tetrafluoroborate.
Structure of Dinitrogen Pentoxide:
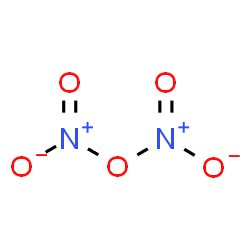
The crystal structure of dinitrogen pentoxide is hexagonal. Its molecular shape is flat. It is a binary compound with two elements as nitrogen and oxygen. It is a solid, but sublimes into a colorless gas when the temperature is raised above room temperature.
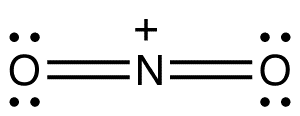
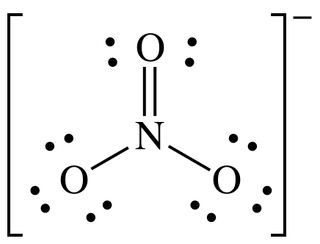
Solid nitrogen pentoxide is a salt. It has separate cations and anions. The cation (NO2+) of nitronium ion has a linear structure, whereas the anion (NO3–) is planar. Therefore, solid nitrogen pentoxide can also be referred to as nitronium nitrate. The oxidation state of the Nitrogen center is +5.
| Total number of atoms in structure | 7 |
|---|---|
| Total number of Valence electrons | 30 + 10 = 40 |
| Total number of Electrons electrons required for Octet | 7 x 8 = 56 |
| Missing electrons required for Octet | 56 – 40 = 16 |
| Number of Bonds | 16 ÷ 2 = 8 |
Preparation of Dinitrogen Pentoxide:
N2O5 is a dehydrate of HNO3. So we can use dehydrating agents like P4O10 to dehydrate HNO3. N2O5 is produced when P4O10 reacts with HNO3 (nitric acid) to give N2O5 and H3PO4. The balanced chemical reaction is given as:.
P4O10 + 12HNO3 → 4H3PO4 + 6N2O5
Physical Properties of Dinitrogen Pentoxide:
- N2O5 is a colorless crystalline solid. However, it cannot be stored at room temperature (at least in India) as it has a melting point of 30°C.
- It turns to a yellow liquid when melted at 30°C.
- You should be careful when handling it as it decomposes when heated above 30°C, but decomposes with an explosion.
- When N2O5 is heated, it dissociates into nitrogen dioxide (NO2) and oxygen (O2).
- N2O5 is an acid anhydride. Anhydride is a compound formed after removing a water molecule from a larger compound. So anhydrous basically means no water.
- N2O5 exists as HNO3 without water. Since HNO3 or nitric acid is an acid, dinitrogen pentoxide is also an acid.
Chemical Properties of Dinitrogen Pentoxide:
- Nitrogen pentoxide reacts with water to form nitric acid. Its balanced chemical equation is:
N2O5 + H2O → 2HNO3
- Nitrogen pentoxide reacts with a base such as sodium hydroxide to form sodium nitrate and water. Its balanced chemical equation is:
N2O5 + 2NaOH → 2NaNO3 + H2O
Uses of Dinitrogen Pentoxide:
- Used as a stable oxidizer in high fuel rockets.
- Used in non-aqueous solvents so that highly water-sensitive molecules can easily nitrate.
- Used as a nitrifying agent in modern synthetic organic chemistry. A mixture of N2O5 and HNO3 is also a good and strong nitrifying agent.
- Used to indicate the intensity of transmitted light when cells are occupied by decomposing nitric pentoxide and when cells are occupied by completely degraded nitric pentoxide, respectively.
Important Key Points:
- Nitrogen pentoxide is colorless.
- It remains solid up to 30°C. Then it starts to melt. It explodes at 40°C and decomposes into NO2 and O2..
- N2O5 is nitric acid in its dehydrated form.
- Adding water can turn it back into nitric acid.
- It is known as anhydrous because it does not contain water.
Frequently Asked Questions – FAQs:
- How can you prepare Nitrogen Pentoxide?
The chemical formula for nitrogen pentoxide is N2O5. N2O5 can be obtained by dehydrating nitric acid to phosphorus pentoxide. Nitrogen pentoxide is primarily used as a nitrifying agent. It can also be used to make explosives. However, people rarely use N2O5 today.The reaction is:
P4O10 + 12HNO3 → 4H3PO4
- What is the use of Nitrogen Pentoxide?
Nitrogen pentoxide is primarily used as a nitrifying agent. It can also be used to make explosives. However, people rarely use N2O5 today.
- Is N2O5 ionic or covalent bond?
N2O5 is composed of two non-metals: oxygen and nitrogen. The ionic charge of oxygen is -2 and the ionic charge of nitrogen is -3. When two non-metals or two negatively charged atoms try to form a bond, they always form a covalent bond. Therefore, nitric pentoxide is not ionic but covalent.
- Is N2O5 acidic or basic?
Nitric pentoxide is acidic. It is formed by dehydration of nitric acid. So adding water will give you back HNO3. Therefore, nitric pentoxide is acidic.