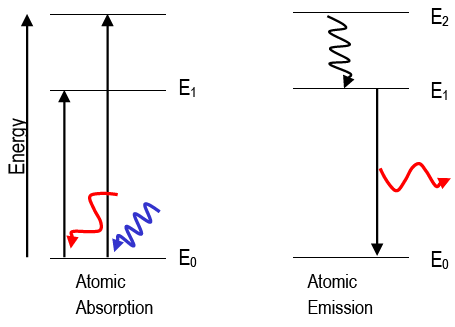
When the light that has the property of wave considered electromagnetic radiation travels in space with the speed of light. It is characterized by the range of wavelength, frequency, and energy of photons. When we study the interaction of matter with electromagnetic radiation either absorption or emission of energy by the atoms or molecules of the sample takes place. Absorption and emission spectra are two types of spectra that are used to investigate the behaviour of light as it interacts with matter.
Introduction
Electromagnetic radiation consists of a wide range of the spectrum that has characteristics of frequency, wavelength, and photon energy when passed through a sample, determines how much light is absorbed in each wavelength. This is known as an absorption spectrum. Some of the light that travels through a sample may be absorbed by its atoms or molecules, leading the electrons in those particles to shift to higher energy levels. The amount of light absorbed at each wavelength is then plotted to generate the absorption spectrum. Atomic and molecular absorption spectra are just two examples of the different types of absorption spectra. When light is absorbed by individual atoms, atomic absorption spectra are created, whereas molecular absorption spectra are created when light is absorbed by molecules. A spectrophotometer can be used to determine a substance’s absorption spectra by counting how much light is absorbed by it at various wavelengths.
An emission spectrum, on the other hand, is a range of electromagnetic radiation that a sample emits when excited, such as when it is heated or exposed to an electric current. When atoms or molecules are stimulated, they can release energy in the form of light at specified wavelengths. The intensity of light emitted at each wavelength is measured to produce the emission spectrum. Atomic emission spectra and molecular emission spectra are two examples of the various forms of emission spectra. Molecular emission spectra are created when molecules emit light as their electronic or vibrational states change, whereas atomic emission spectra are created when atoms emit light when they transition from excited to their ground state. A spectroscope can be used to measure an object’s emission spectra since it divides the light being emitted into its various wavelengths and makes it possible to detect the object’s emission lines.
A sample’s atoms or molecules can be identified and their energies and attributes can be determined using both the absorption and emission spectra. Each element or compound’s specific pattern of lines in these spectra can be used as a “fingerprint” to identify it. To better comprehend the behaviour of light and to evaluate the characteristics of matter, these spectra are frequently utilized in the sciences of chemistry, astronomy, and physics. For example, in chemistry, unknown substances are identified by comparing their absorption or emission spectra to those of recognized substances. The composition and distance of stars and galaxies are found by looking at their absorption or emission spectra in astronomy.
Difference between Emission and Absorption Spectra
The interaction of electromagnetic radiation with matter, absorption and emission spectra are complementary approaches that are utilized in different ways depending on their processes, energies, and purposes.
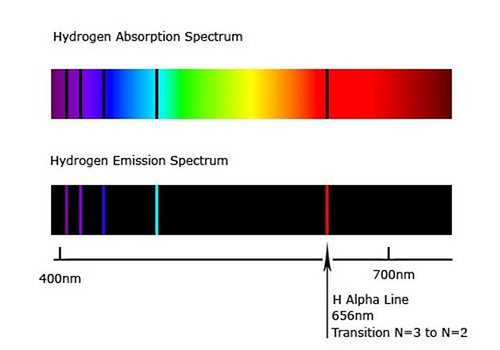
Atomic absorption and emission spectrum of Hydrogen Atom.
| Emission Spectra | Absorption Spectra |
|---|---|
| Emission spectra describe the pattern of electromagnetic radiation a substance emits. | Absorption spectra describe the pattern of electromagnetic radiation a substance absorbs. |
| When a substance is activated by an outside energy source and transitions back to its ground state, it emits electromagnetic radiation in emission bands. | In absorption spectra, electromagnetic radiation travels through a substance and is partially absorbed by it, resulting in a reduction in the radiation’s intensity. |
| Emission spectra are created when a substance emits excess energy in the form of electromagnetic radiation as its electrons return higher to lower energy levels. | Absorption spectra are created when a substance absorbs specific wavelengths of light to excite its electrons from lower to higher energy levels. |
| Emission spectra are used to identify the content and characteristics of a substance or material, such as in the study of stars, galaxies, and chemical compounds. | Absorption spectra are used to detect the presence of a substance in a sample and to calculate its concentration. |
| Emission spectra are measured using a spectroscope, which breaks down the emitted light into its various wavelengths. | Absorption spectra are measured using a spectrophotometer, which counts the amount of light absorbed by the substance at various wavelengths. |
| It comprises colored lines in the spectrum as shown in Fig2. | It comprises dark lines or gaps in the spectrum as shown in Fig2. |
Recommended Articles:
Difference Between Earth And Neutral
Difference Between Earthing And Grounding
Difference Between Electric Field And Magnetic Field
Difference between Electromagnet and Permanent Magnet
Difference between EMF and Voltage
When energy is absorbed by electrons in the ground state to reach higher energy levels, the frequencies of light transmitted with dark bands are known as the absorption spectrum. An atom's electrons move from lower energy levels to higher energy levels when energy is absorbed by them. These excited electrons must emit energy in order to leave their unstable excited state and return to their ground states. The frequencies of this released light create the emission spectrum Absorption spectra are measured using a spectrophotometer, which counts the amount of light absorbed by the substance at various wavelengths. Emission spectra are measured using a spectroscope, which breaks down the emitted light into its various wavelengths. Difference between Emission and Absorption Spectra FAQs
What is the Absorption Spectra?
What is the Emission spectra?
Name the device used to measure absorption spectra.
Name the device used to measure emission spectra.