Hydrocarbons yield new compounds like alcohols and phenols through hydrogen atom replacement. Alcohols have -OH and phenols are in aromatic hydrocarbons. They’re used in daily life, such as ethanol as a polish. -OH groups are present in essential items like food, clothing, and paper.
Introduction
Alcohols and Phenols are organic compounds with -OH groups that have unique properties. Alcohols have an -OH group attached to a carbon in an aliphatic chain and are polar, forming hydrogen bonds, making them water-soluble. Phenols have an -OH group attached to an aromatic ring, making them more reactive than alcohol.
What is Alcohol?
Alcohols have a hydroxyl (-OH) group attached to a carbon atom in an aliphatic chain. They are typically represented by the formula R-OH, where R is an alkyl group. Alcohols are polar compounds and have a tendency to form hydrogen bonds, making them soluble in water. They are commonly used as solvents, fuel, and in the production of personal care and pharmaceutical product.
What is Phenol?
Phenols, on the other hand, have a hydroxyl (-OH) group attached to an aromatic ring. They are represented by the formula Ar-OH, where Ar is an aromatic ring. Phenols are more polar than alcohols and have stronger hydrogen bonding properties. This makes them even more soluble in water.
Structure of Alcohol and Phenol
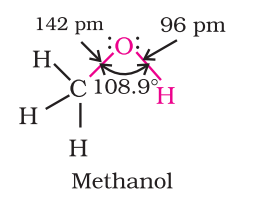
- Alcohol: The connection between the oxygen atom in the -OH group and the carbon atom in alcohols is a sigma bond, which is created by the combination of a sp3 hybridized orbital from carbon and a sp3 hybridized orbital from oxygen. The bond angle in alcohols is slightly smaller than the tetrahedral angle of 109°-28′ because of the interference between the unshared electron pairs of oxygen.
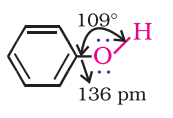
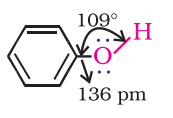
- Phenol: The hydroxyl group (-OH) in phenols is linked to the sp2-hybridized carbon atom in an aromatic ring. The carbon-oxygen bond length in phenols, at 136 pm, is shorter compared to that in methanol. This is attributed to the double bond-like character in the bond due to conjugation of the oxygen’s unshared electron pair with the aromatic ring, as well as the sp2-hybridization state of the carbon to which oxygen is bound.
Properties of Phenol and Alcohol
Alcohols and phenols differ by alkyl/aryl group but have common hydroxyl group. Boiling points increase with more carbon atoms due to stronger van der Waals forces.
- In alcohols, the boiling points decrease when the carbon chain becomes more branched because the van der Waals forces are reduced and the surface area decreases.
- The hydroxyl (-OH) group in alcohols and phenols participates in intermolecular hydrogen bonding. Thus, boiling points of alcohols and phenols are higher than similar compounds.
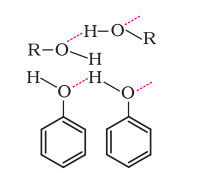
- The solubility of alcohols and phenols in water is a result of their capability to engage in hydrogen bonding with water molecules.
- Alcohols are highly adaptable substances that have the ability to react as both nucleophiles and electrophiles. When they react as nucleophiles, the O-H bond in the hydroxyl group is broken.
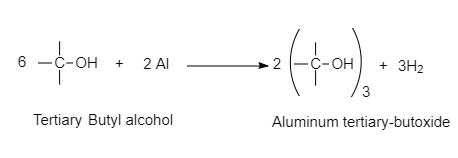
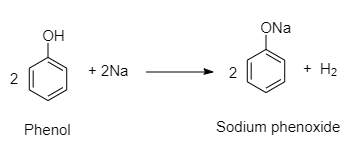
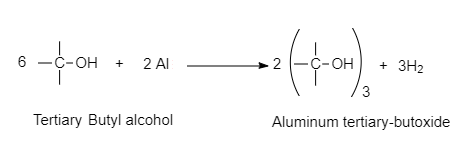
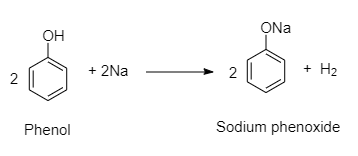
For example, Metals such as sodium, potassium and aluminum can react with alcohols and phenols to produce alkoxides/phenoxides and hydrogen.
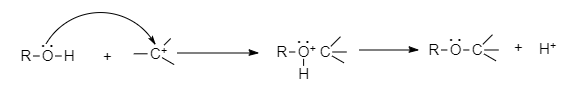
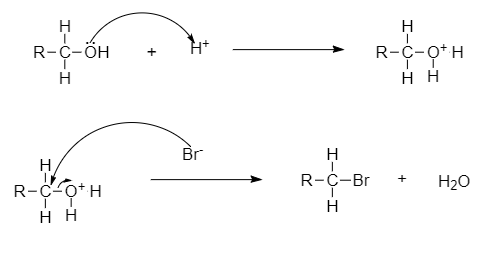
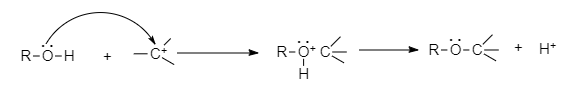
- The C-O bond can be broken in reactions with electrophiles, as demonstrated by protonated alcohols.
For example, Alkyl halide to haloalkane can be converted through this mechanism.
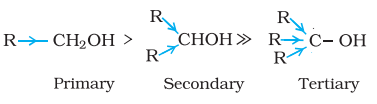
- The acidity of alcohols is a result of the polar O-H bond. When an electron-donating group, such as -CH3 or -C2H5, is present, it increases the electron density on the oxygen atom, reducing the polarity of the O-H bond and thus lowering the level of acidity. As a result, the acidity of alcohols decreases in the following sequence:
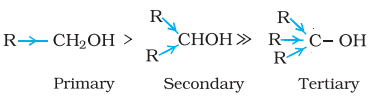
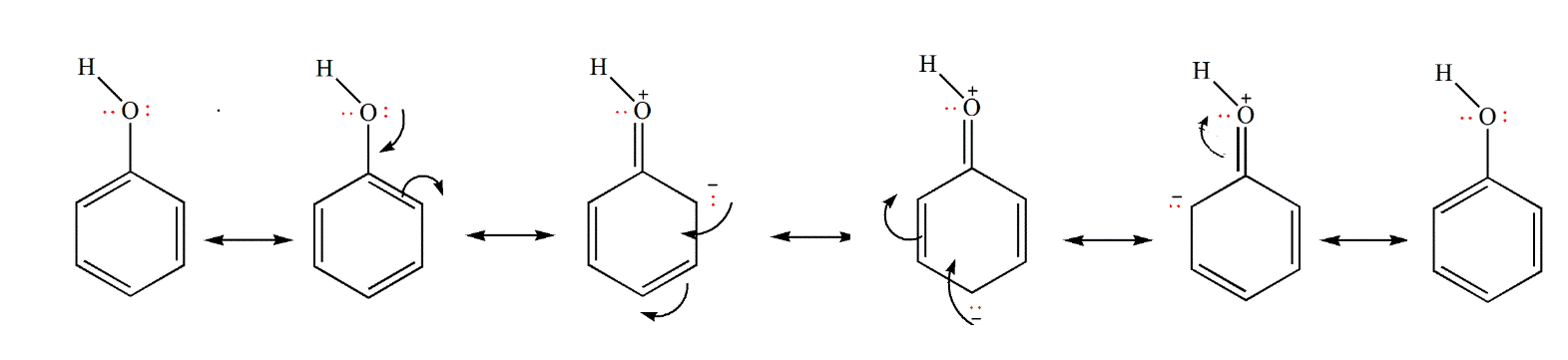
- Phenols have strong acidic behavior due to a positively charged oxygen in the -OH group. This is due to the bond between the hydroxyl group and a sp2 hybridized carbon in the benzene ring acting as an electron-withdrawing group, as seen in the molecule’s resonance structures, and more stable delocalized negative charge in phenoxide ion, leading to greater ionization.
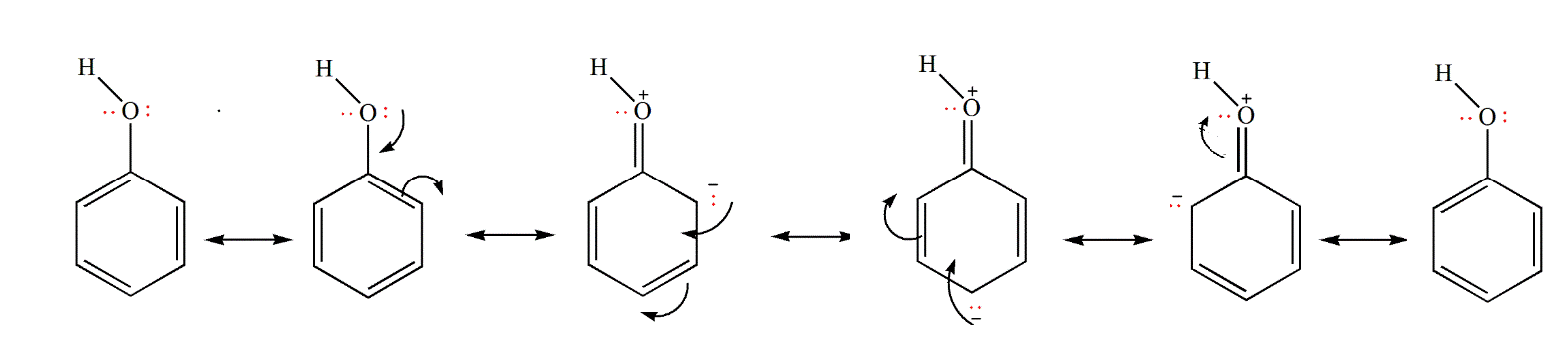
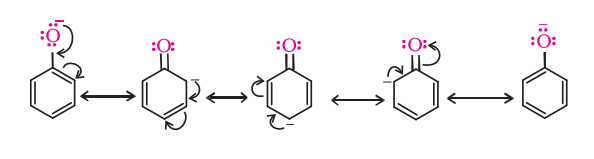
Differences between Alcohol and Phenol
| Alcohol | Phenol |
|---|---|
| Alcohols have a hydroxyl group (-OH) attached to a carbon atom in an alkyl chain. | Phenols have the hydroxyl group attached to a carbon atom in an aromatic ring. |
| Alcohols are weaker acids than phenol. Due to decreased polarity of the O-H bond. | Phenols are stronger acids than alcohols due to increased polarity and delocalized negative charge. |
| Alcohols have lower O-H bond polarity and acidity due to lower electronegativity of carbon. | The electronegativity of the carbon atom to which the hydroxyl group is attached is higher in phenols, leading to a higher polarity of the O-H bond. |
| Alcohols are less soluble in water than phenols due to their polar nature and larger hydrocarbon part in their structue. | Phenols are more soluble in water than alcohols due to their higher polar nature. |
| Alcohols do not have any electron rich aromatic ring in their structure and this making them less reactive. | Phenols are more reactive than alcohols due to the presence of the aromatic ring, which makes them more susceptible to electrophilic substitution reactions. |
Frequently Asked Questions(FAQs)
- What is Alcohol?
Alcohols are compounds that have a hydroxyl (-OH) group attached to a carbon atom in an aliphatic chain. They are polar and soluble in water, used as solvents, fuel, and in personal care and pharma production.
- What is Phenol?
Phenols are compounds with a hydroxyl (-OH) group attached to an aromatic ring. They are more polar and highly soluble in water, used in antiseptics, analgesics, and plastics/resins production.
- How are Alcohols and Phenols Structured?
Alcohols have an -OH group linked to a carbon in an aliphatic chain while phenols have an -OH group linked to a carbon in an aromatic ring.
- Mention few examples of alcohol and phenol?
Examples of alcohols are Ethanol, Propanol, Butanol and Examples of phenols Cresol, Resorcinol, Catechol, etc.
Difference Between Alcohol And Phenol
Hydrocarbons yield new compounds like alcohols and phenols through hydrogen atom replacement. Alcohols have -OH and phenols are in aromatic hydrocarbons. They’re used in daily life, such as ethanol as a polish. -OH groups are present in essential items like food, clothing, and paper.
Introduction
Alcohols and Phenols are organic compounds with -OH groups that have unique properties. Alcohols have an -OH group attached to a carbon in an aliphatic chain and are polar, forming hydrogen bonds, making them water-soluble. Phenols have an -OH group attached to an aromatic ring, making them more reactive than alcohol.
What is Alcohol?
Alcohols have a hydroxyl (-OH) group attached to a carbon atom in an aliphatic chain. They are typically represented by the formula R-OH, where R is an alkyl group. Alcohols are polar compounds and tend to form hydrogen bonds, making them soluble in water. They are commonly used as solvents, fuel, and in the production of personal care and pharmaceutical product.
What is Phenol?
Phenols, on the other hand, have a hydroxyl (-OH) group attached to an aromatic ring. They are represented by the formula Ar-OH, where Ar is an aromatic ring. Phenols are more polar than alcohols and have stronger hydrogen bonding properties. This makes them even more soluble in water.
Structure of Alcohol and Phenol
- Alcohol: The connection between the oxygen atom in the -OH group and the carbon atom in alcohols is a sigma bond, which is created by the combination of a sp3 hybridized orbital from carbon and a sp3 hybridized orbital from oxygen. The bond angle in alcohols is slightly smaller than the tetrahedral angle of 109°-28′ because of the interference between the unshared electron pairs of oxygen.
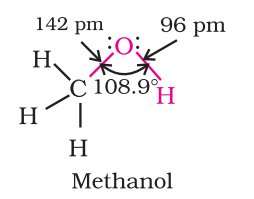
- Phenol: The hydroxyl group (-OH) in phenols is linked to the sp2-hybridized carbon atom in an aromatic ring. The carbon-oxygen bond length in phenols, at 136 pm, is shorter compared to that in methanol. This is attributed to the double bond-like character in the bond due to conjugation of the oxygen’s unshared electron pair with the aromatic ring, as well as the sp2-hybridization state of the carbon to which oxygen is bound.
Properties of Phenol and Alcohol
Alcohols and phenols differ by alkyl/aryl group but have common hydroxyl group. Boiling points increase with more carbon atoms due to stronger van der Waals forces.
- In alcohols, the boiling points decrease when the carbon chain becomes more branched because the van der Waals forces are reduced and the surface area decreases.
- The hydroxyl (-OH) group in alcohols and phenols participates in intermolecular hydrogen bonding. Thus, boiling points of alcohols and phenols are higher than similar compounds.
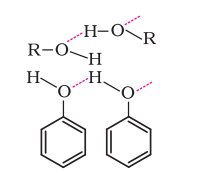
- The solubility of alcohols and phenols in water is a result of their capability to engage in hydrogen bonding with water molecules.
- Alcohols are highly adaptable substances that have the ability to react as both nucleophiles and electrophiles. When they react as nucleophiles, the O-H bond in the hydroxyl group is broken.
For example, Metals such as sodium, potassium and aluminum can react with alcohols and phenols to produce alkoxides/phenoxides and hydrogen.
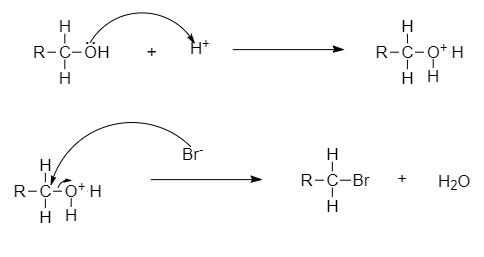
- The C-O bond can be broken in reactions with electrophiles, as demonstrated by protonated alcohols.
For example, Alkyl halide to haloalkane can be converted through this mechanism.
- The acidity of alcohols is a result of the polar O-H bond. When an electron-donating group, such as -CH3 or -C2H5, is present, it increases the electron density on the oxygen atom, reducing the polarity of the O-H bond and thus lowering the level of acidity. As a result, the acidity of alcohols decreases in the following sequence:
- Phenols have strong acidic behavior due to a positively charged oxygen in the -OH group. This is due to the bond between the hydroxyl group and a sp2 hybridized carbon in the benzene ring acting as an electron-withdrawing group, as seen in the molecule’s resonance structures, and more stable delocalized negative charge in phenoxide ion, leading to greater ionization.
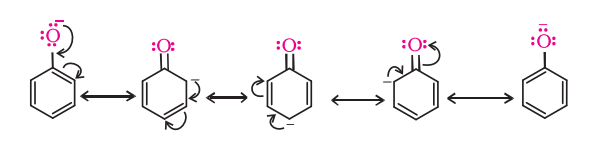
Differences between Alcohol and Phenol
| Alcohol | Phenol |
|---|---|
| Alcohols have a hydroxyl group (-OH) attached to a carbon atom in an alkyl chain. | Phenols have the hydroxyl group attached to a carbon atom in an aromatic ring. |
| Alcohols are weaker acids than phenol. Due to decreased polarity of the O-H bond. | Phenols are stronger acids than alcohols due to increased polarity and delocalized negative charge. |
| Alcohols have lower O-H bond polarity and acidity due to lower electronegativity of carbon. | The electronegativity of the carbon atom to which the hydroxyl group is attached is higher in phenols, leading to a higher polarity of the O-H bond. |
| Alcohols are less soluble in water than phenols due to their polar nature and larger hydrocarbon part in their structue. | Phenols are more soluble in water than alcohols due to their higher polar nature. |
| Alcohols do not have any electron rich aromatic ring in their structure and this making them less reactive. | Phenols are more reactive than alcohols due to the presence of the aromatic ring, which makes them more susceptible to electrophilic substitution reactions. |
Frequently Asked Questions(FAQs)
- What is Alcohol?
Alcohols are compounds that have a hydroxyl (-OH) group attached to a carbon atom in an aliphatic chain. They are polar and soluble in water, used as solvents, fuel, and in personal care and pharma production.
- What is Phenol?
Phenols are compounds with a hydroxyl (-OH) group attached to an aromatic ring. They are more polar and highly soluble in water, used in antiseptics, analgesics, and plastics/resins production.
- How are Alcohols and Phenols Structured?
Alcohols have an -OH group linked to a carbon in an aliphatic chain while phenols have an -OH group linked to a carbon in an aromatic ring.
- Mention few examples of alcohol and phenol?
Examples of alcohols are Ethanol, Propanol, Butanol and Examples of phenols Cresol, Resorcinol, Catechol, etc.