Introduction
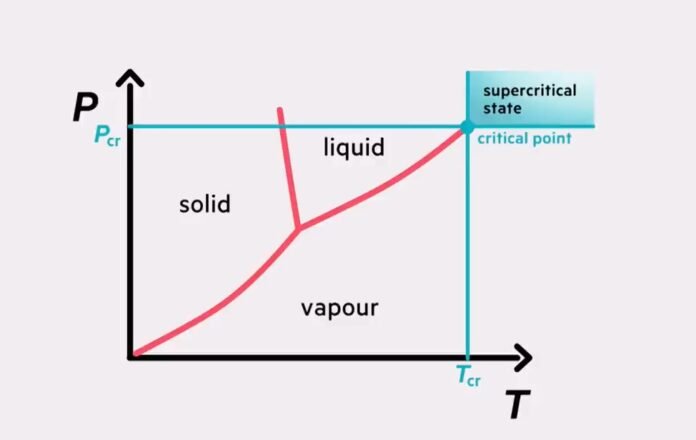
It is the pressure required to liquefy a gas at its critical temperature, which is the highest temperature at which the gas can be condensed into a liquid by increasing pressure alone. Above the critical temperature, the gas cannot be liquefied by increasing pressure alone, no matter how high the pressure is.
Triple Point
- To understand critical pressure, it is essential to understand the concept of the triple point.
- The triple point is the point at which a substance’s solid, liquid, and gas phases coexist in equilibrium. At this point, the pressure and temperature are such that the substance can exist in all three phases simultaneously.
- The triple point is a crucial concept in thermodynamics, defining the thermodynamic temperature scale. The triple point of water is 0.01 degrees Celsius.
What is Critical Pressure?
- The critical pressure is the pressure at which a substance undergoes a phase transition from a gas to a liquid state. At this point, the substance is said to be in its critical state.
- The critical point is the point at which a substance’s liquid and gas phases become indistinguishable. At this point, the substance has a critical temperature, critical pressure, and critical density.
Critical Pressures of Some Common Substances
The critical pressure of a substance depends on its molecular structure and the intermolecular forces that hold the molecules together.
Some common substances and their critical pressures are listed below:
| Substance | Critical Pressure |
|---|---|
| Water | 220.6 atm |
| Carbon dioxide | 73.8 atm |
| Nitrogen | 33.5 atm |
| Methane | 45.8 atm |
| Propane | 42.5 atm |
| Ethane | 48.8 atm |
| Hydrogen | 12.9 atm |
Applications of critical pressure
Gas Chromatography
- Gas chromatography is a technique used to separate and analyse the components of a mixture. The critical pressure is an essential parameter in gas chromatography, as it affects the selectivity and efficiency of the separation.
Supercritical Fluid Extraction
- Supercritical fluid extraction extracts and purifies compounds from a mixture using supercritical fluids, such as carbon dioxide. The critical pressure is an essential parameter in supercritical fluid extraction.
Industrial Applications
- The concept of critical pressure is used in several industrial applications, such as the production of liquefied natural gas (LNG). The production of LNG involves cooling natural gas to its critical temperature and pressure, which results in the condensation of the gas into a liquid.
Environmental applications
- Critical pressure is important in environmental applications such as carbon capture and storage. In this process, carbon dioxide is compressed and stored underground at high pressures and low temperatures. It becomes a supercritical fluid with properties that make it easier to store and transport.
Recommended Articles:
Conventional Methods Measurements: Time, Length, And Weight
Convex Lens: Introduction, Difference, Uses, & Types
Convex Mirror: Introduction, Works, Formation, Properties, And Uses
Coriolis Effect: Introduction, Causes, Demonstration, And Characteristics
Coriolis Effect: Introduction, Effect, Derivation, Characteristics, And Significance
Critical pressure is the pressure at which a substance undergoes a phase transition from a gas to a liquid state. It is an essential parameter in thermodynamics that describes the behavior of fluids under varying temperature and pressure conditions. The critical point of a substance is the point at which the liquid and gas phases become indistinguishable. The critical point is defined by the critical pressure, critical temperature, and critical density of a substance. Supercritical fluid extraction is a technique that involves the use of a supercritical fluid to extract compounds from a mixture with high selectivity and efficiency. The critical pressure is an important parameter in this process as it affects the solubility and selectivity of the extraction. To produce LNG, gas is cooled to its critical temperature/pressure, causing it to condense into liquid. Critical pressure is crucial, as it determines the liquefaction conditions. The behavior of materials near the critical point is strongly influenced by the critical pressure, critical temperature, and critical density. Controlling the critical pressure makes it possible to tailor the properties of materials such as polymers and liquid crystals to meet specific application Critical Pressure FAQs
What is critical pressure?
What is the critical point of a substance?
What is the significance of critical pressure in supercritical fluid extraction?
How is critical pressure used to produce liquefied natural gas (LNG)?
What is the role of critical pressure in materials science?