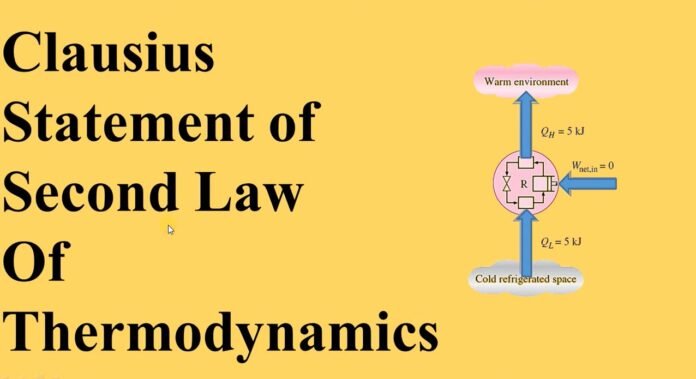
It is very impossible to form a device that operates on a cycle and whose effect is the heat transfer from a body which was cooler to a hotter one.There is a different way to explain the Second law of thermodynamics.
Clausius statement is one of the best way to explain the second law of thermodynamics.In this article we will discuss about the clausius statement, Clausius theorem, Second Law of Thermodynamics and its application
Clausius Statement
By the 2nd law of thermodynamics Clausius statement states that –
“It is impossible to create a device that operates on a cycle and has no other consequences than to transfer the heat from a cooler body( low Temperature) to a hotter body (High Temperature).”
Clausius showed that when some quantity of heat q is introduced to a system at the absolute temperature T, then the system would undergo a process and that the ratio Q/T is the same for all reversible processes. He calculated the value which is given as (dS = Q/T) here, S as the entropy.
Who was Rudolf Clausius
The full name of Rudolf Clausius is Rudolf Julius Emanuel Clausius. Rudolf Clausius was a physicist and German mathematician who was responsible for the development of thermodynamics and the Clausius assertion.
He found a lot of ideas about heat and energy and how heat and properties work, which is a restatement of the Carnot cycle.
Kelvin Planck Statement
“When we give some amount of heat to a system then a system cannot provide the same amount of work output as it receives from a high-temperature reservoir”.
While a system that converts the amount of work into heat is possible, a device that converts heat back into work is not viable.
Thermal efficiency cannot be 100% with a heat engine, on the other hand.
Second Law of Thermodynamics
The second law of thermodynamics deals with the transfer of heat naturally from a hot body to a cold body.
Without the addition of energy the heat energy cannot transfer from a body at a lower temperature to a body at a higher temperature.
The First Law of Thermodynamics states that energy can neither be created nor be destroyed it can transfer from one body to another
The total amount of energy available to be used by all things in the universe remains conserved. The 2nd Law of Thermodynamics is related to the nature of energy and how heat and energy can be transformed.
Application of Second Law of Thermodynamics
It states that heat always moves from a body at high temperature to a body at low temperature.
Refrigerators and heat pumps are work on the second law of thermodynamics which is based on the Reversed Carnot cycle
Recommended Articles:
Circuit Diagram: Introduction, Example,Types, & Circuit Diagram
Class 8 Physics Index
Class 11 Physics Index
Class 12 Physics Index: Contents, Importance, and FAQs
Classification Of Polymerization: Introduction, Classification, And FAQs
Clausius statement – It states that it is impossible to produce a device which operates on a cycle and has no other consequences than to transfer the heat from a cold body (low temperature) to a hot body (high temperature). These are the law of thermodynamics - First law of Thermodynamics The law states that heat always moves from a body whose temperature is high to a body which has a low temperature. According to this law- The heat energy is not transferred from a body having lower temperature to a body having higher temperature without the addition of energy. The Carnot engine is a theoretical thermodynamic cycle. It works on the principle of the Carnot cycle which provides an assessment of the maximum possible efficiency that a heat engine converts heat into output work working between two reservoirs. According to clausius inequality for a close system- the process is irreversible if Tds is greater than dq. According to Clausius it is impossible to create a device that can transfer heat from a cold body to a body. It is said because heat can only be transferred when the temperature decreases. Clausius Statement FAQs
What is Clausius Statement?
What are the four laws of thermodynamics?
Second law of Thermodynamics
Third law of Thermodynamics
Zeroth law of ThermodynamicsWrite one application of the Second Law of thermodynamics.
What is the second law of thermodynamics?
What is a Carnot Engine?
What is the clausius inequality statement?
What is the importance of clausius statement?