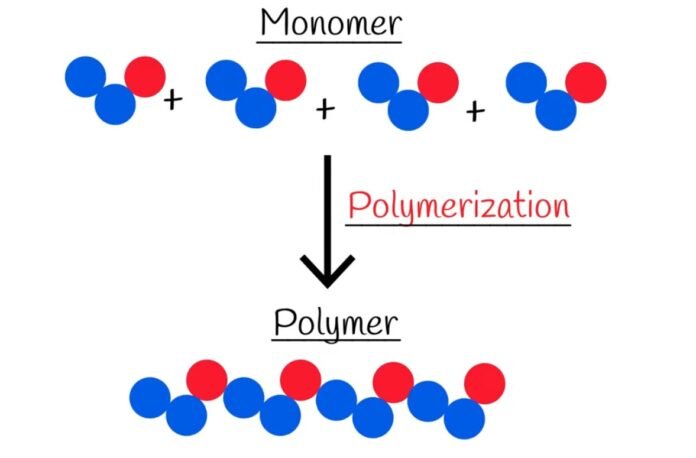
Polymerization is a process that occurs in chemical compounds and can happen through various reaction mechanisms, which can be simple or complex depending on the functional groups present in the reactants and their influence on the reaction. Alkenes can easily form polymers through straightforward radical reactions. However, reactions that involve substitution at a carbonyl group are more complicated to synthesize. Alkanes can also undergo polymerization but only with the aid of strong acids.
Introduction
Polymerization is a chemical process in which small molecules called monomers are combined to form large molecules known as polymers. The process of polymerization can be classified into various categories based on the type of reaction involved, the mechanism of the reaction, the monomers involved, and the end product.
Classification
Origin
Natural Polymers
Natural polymers are polymers that are found in nature, such as proteins, carbohydrates, and nucleic acids. For example, Proteins (Hemoglobin, collagen), Carbohydrates (Starch, cellulose), and Nucleic acids (DNA, RNA). 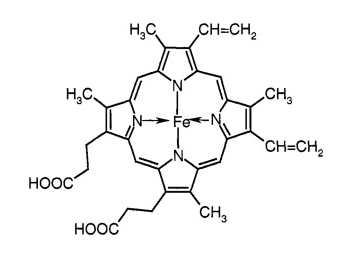
Semi-Synthetic polymers
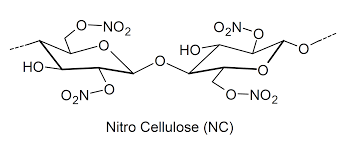
Semi-synthetic polymers are those that are produced by modifying natural polymers. They are a combination of natural and synthetic materials. Examples are cellulose acetate, nitrocellulose, and rayon.
Synthetic polymers
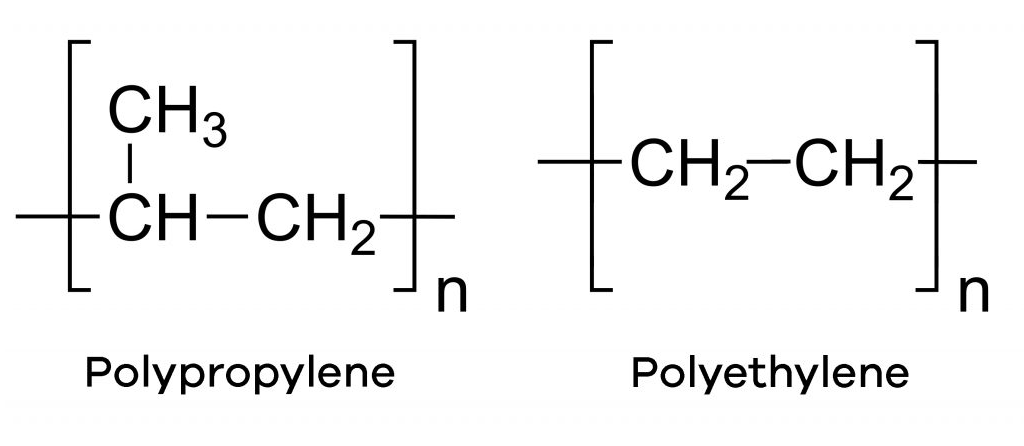
Synthetic polymers, on the other hand, are artificially created polymers that do not exist in nature. Examples include Polypropylene, Polyethylene, Polyvinyl chloride (PVC), etc.
Polymer Structure
Linear Polymers
Linear polymers are those in which the polymer chains are straight and unbranched, resulting in a long, continuous chain. Linear polymers have a uniform molecular weight distribution and exhibit simple, predictable physical and mechanical properties. Examples of linear polymers include polyethylene and polystyrene.
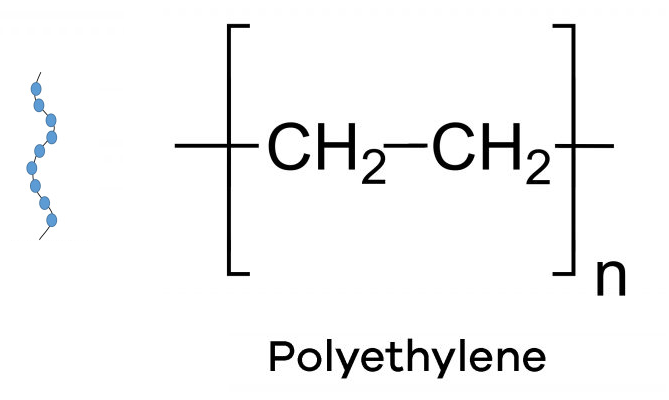
Crosslinked Polymers
Crosslinked polymers are those in which the polymer chains are connected to each other by covalent bonds, forming a three-dimensional network. Crosslinking creates a more rigid, thermoset structure, with improved heat resistance and mechanical strength compared to linear polymers. Examples of crosslinked polymers include epoxy, polyester fiberglass, and silicone.
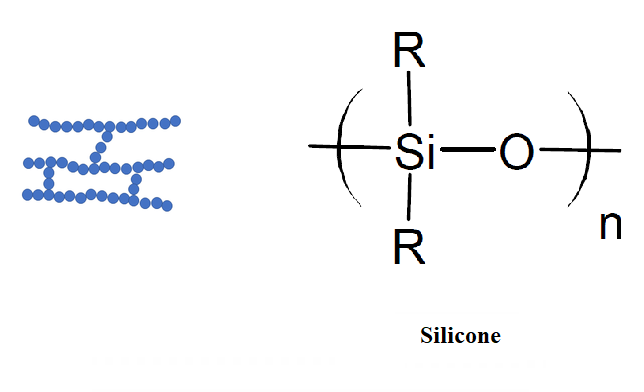
Branched Polymers
Branched polymers are those in which the polymer chains contain branches, or side chains, that extend from the main chain. Branched polymers exhibit a more complex molecular weight distribution than linear polymers and can have a variety of properties depending on the size and distribution of the branches. Examples of branched polymers include low-density polyethylene (LDPE) and polybutadiene.
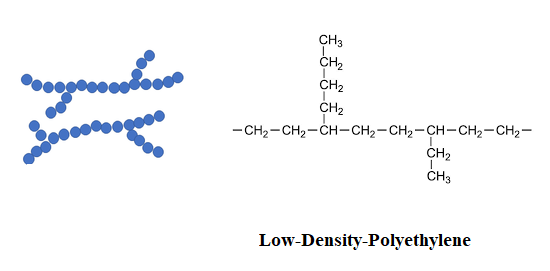
Polymerization reaction
Addition Polymerization
Addition polymerization is a type of reaction in which monomers are joined together by adding functional groups to form a polymer chain. The reaction typically occurs through a free radical mechanism. Examples of addition polymerization include the polymerization of ethene to form polyethylene and the polymerization of styrene to form polystyrene.
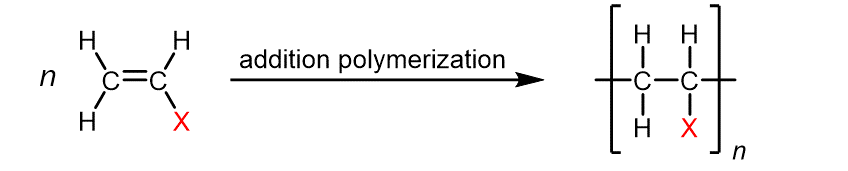
Condensation Polymerization
Condensation polymerization is a type of reaction in which monomers are joined together by losing a small molecule, such as water or methanol, during the reaction. This type of polymerization reaction is typically exothermic and results in the formation of a polymer chain and a small molecule by-product. Examples of condensation polymerization include the polymerization of Terephthalic acid and ethylene glycol. The concentration of monomer we use in the polymerization results in either Polyethylene terephthalate or BHET.
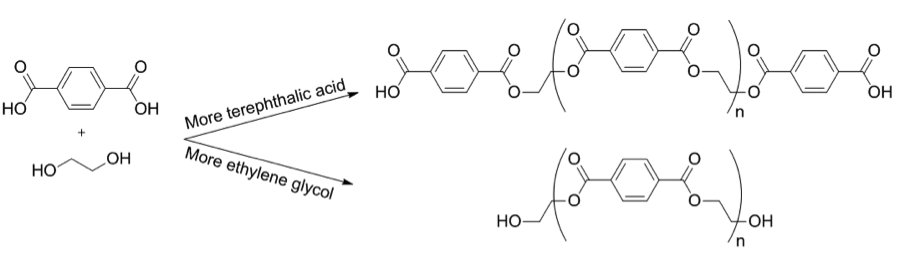
Polymerization reaction mechanism
Step-growth Polymerization
Step-growth polymerization is a method of polymerization where bi-functional or multi-functional monomers react to produce dimers, followed by trimers, and longer oligomers, eventually leading to the formation of long-chain polymers. This mechanism is responsible for the creation of many naturally occurring and synthetic polymers, such as polyesters, polyamides, polyurethanes, etc.
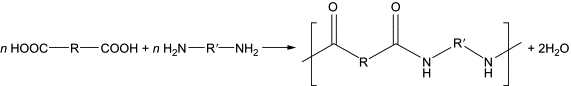
Chain-growth Polymerization
Chain-growth polymerization, also known as chain polymerization, is a process in which the extension of a polymer chain occurs through the addition of a monomer to an active center such as a free radical or ion.
- Radical polymerization:
Free radical polymerization is a type of polymerization mechanism in which a free radical initiates the reaction by breaking a covalent bond in a monomer molecule, creating a growing polymer chain.
The key steps in the free radical chain polymerization mechanism are as follows:
- Initiation: Free radicals are generated by the cleavage of chemical bonds in initiator molecules, For example, Benzoyl peroxide is used as a chain initiator for polyethylene formation.
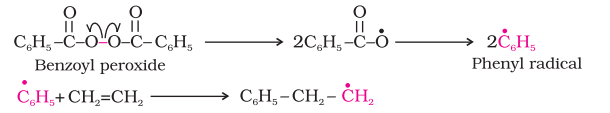
- Propagation: In the propagation step, the phenyl free radicals abstract a hydrogen atom from the ethene monomer to create a new free radical and a growing polymer chain.
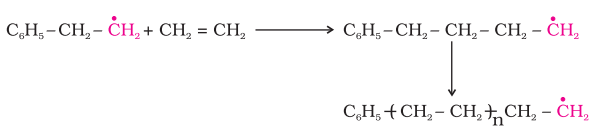
- Termination: The reaction is terminated by the combination of two free radicals, which results in the formation of a non-reactive molecule and the removal of the free radicals from the reaction mixture.
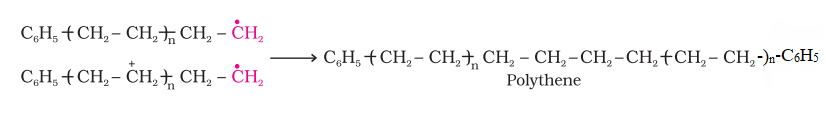
- Ionic polymerization:
Ionic polymerization is a type of polymerization mechanism in which the initiation of polymerization is initiated by the formation of an ionic species (positive or negative), usually from an initiator molecule. For example, Organolithium initiated Polystyrene polymerization, etc.
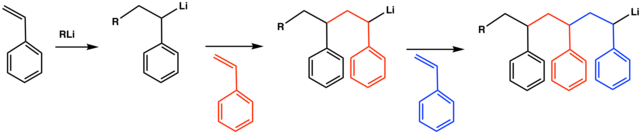
- Ring-Opening Polymerization:
Ring-opening polymerization is a type of reaction in which a cyclic monomer is polymerized to form a linear polymer chain. The reaction typically occurs through an addition reaction mechanism, where a nucleophile attacks the ring of the cyclic monomer, breaking the ring and forming a polymer chain. The reaction can be initiated thermally or through the use of a catalyst. Examples of ring-opening polymerization include the polymerization of cyclic esters, such as lactones to form polyesters.
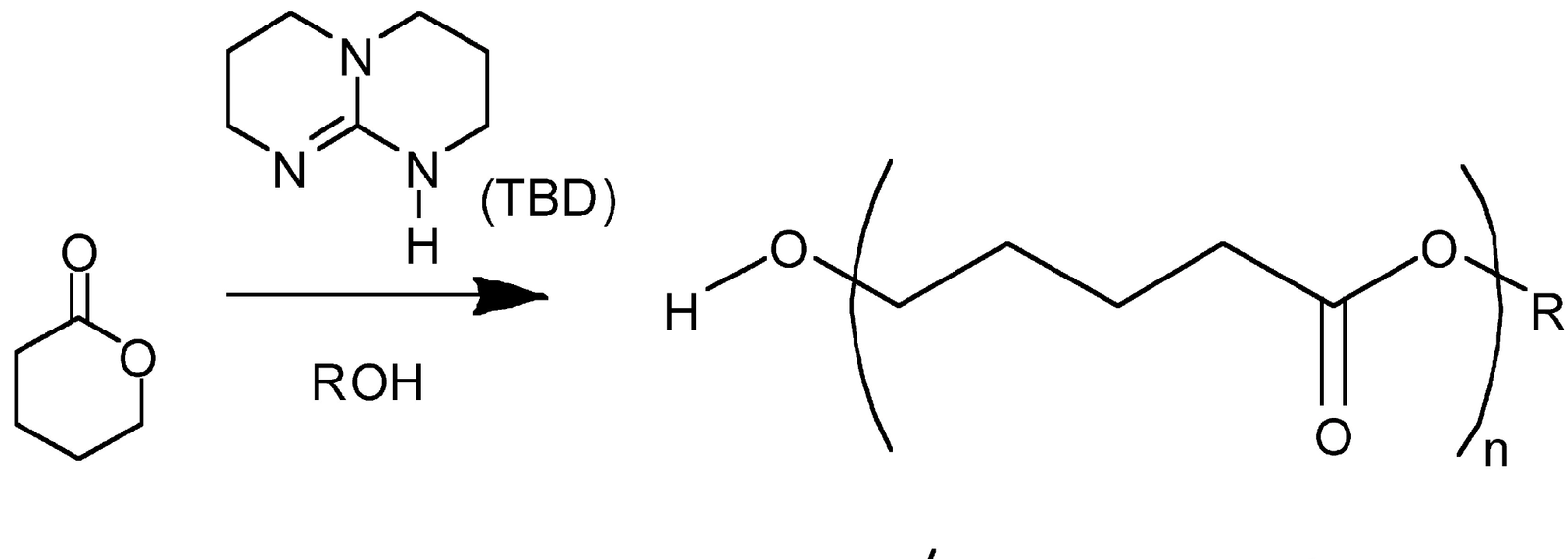
Recommended Articles:
Circuit Component: Active, Source, Transistor, and Components
Circuit Diagram: Introduction, Example,Types, & Circuit Diagram
Class 8 Physics Index
Class 11 Physics Index
Class 12 Physics Index: Contents, Importance, and FAQs
Polymerization is a chemical process in which small molecules (monomers) are combined to form large molecules (polymers). There are two main types of polymerization reactions: addition polymerization and condensation polymerization. Natural polymers are polymers found in nature, such as proteins, carbohydrates, and nucleic acids. Linear polymers have straight, unbranched chains. Crosslinked polymers are connected by covalent bonds, forming a three-dimensional network. Branched polymers contain side chains extending from the main chain.What is polymerization?
What are the types of polymerization reactions?
What are natural polymers?
What is the difference between linear, crosslinked, and branched polymers?