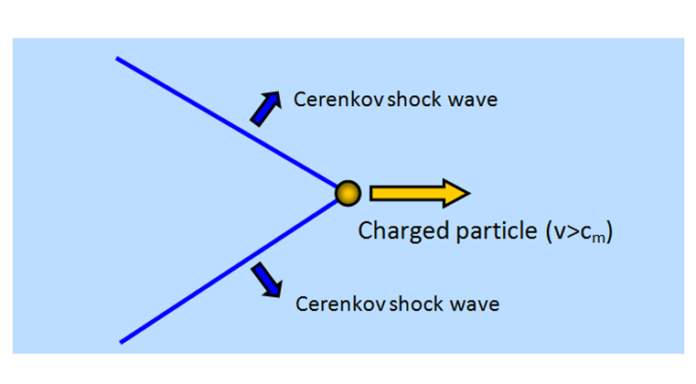
In the realm of cosmology and astronomy, Cherenkov radiation is a form of energy that can be perceived by us as a blue glow emitted when the electrically charged particles that compose atoms (i.e., electrons and protons) are moving at speeds faster than that of light in a specific medium. Cerenkov radiations i.e., CR have been used in various biological research fields.
What are Cherenkov Radiations?
Cherenkov radiation is an electromagnetic radiation named after the Soviet scientist Pavel Cherenkov who was the first to detect it experimentally under the supervision of Sergey Vavilov at the Lebedev Institute in 1934. Due to this reason, this radiation is also known as Vavilov–Cherenkov radiation. These radiations are emitted when a charged particle passes through a dielectric medium at a speed greater than the phase velocity of light in that medium. In 1958, Physics Nobel Prize laureate, Pavel Cherenkov, shared the award with Ilya Frank and Igor Tamm, for being the first to experimentally demonstrate and explain this glow.
When Cherenkov radiation is intense, it appears as a weak bluish-white glow in the pools of water shielding some nuclear reactors. The Cherenkov radiation in cases such as this is caused by electrons from the reactor traveling at speeds greater than the speed of light in water, which is 3/4th of the speed of light in a vacuum. Electrons in some of the atoms along its path are displaced by the energetically charged particle traveling through the medium. The electromagnetic radiations thus emitted combine to form a strong electromagnetic wave analogous to the bow wave caused by a power boat traveling faster than the speed of water waves or to the sonic boom produced by an airplane traveling faster than the speed of sound in air.
Properties of Cherenkov Radiations
This radiation has a high frequency and is continuous and due to its continuity, it does not have any characteristic peaks in its spectrum and appears constant. Due to its high frequencies, it has short wavelengths and is also very intense. It thus emits blue light that falls in the UV i.e., ultraviolet region of the electromagnetic spectrum. It becomes visible to the naked eye due to sufficient accelerated charged particles.
Cherenkov Detectors
A Cherenkov detector is a particle detector based on the detection of Cherenkov radiation (UV photons or visible light). Cherenkov radiation is commonly produced in dielectric materials through pair-production electrons and positrons or Compton electrons. The intensity of light produced by this process is much less than that of luminescence i.e., the basis for scintillation detector operation, requiring more sensitive optical photon detection equipment such as low light PMT i.e., photo-multiplier tubes.
Reason for Blue water in a nuclear reactor
When the Cherenkov radiation passes through water, the charged particles travel faster than light. So, the light has a higher frequency and shorter wavelength than usual and due to the presence of light with a shorter wavelength, the light appears blue in color. Now, the question arises that why is there any light at all. The electrons of water molecules are excited by the fast-moving electrons. These excited electrons absorb energy and release it as charged particles i.e., as photons as they return to equilibrium. The shock waves produced by the particles traveling faster than light produce a constructive interference that we see as a glow in this case.
Cherenkov Effect
The Cherenkov effect takes place when a positron or electron travels through a transparent medium at a speed greater than that of light in that medium. This would cause a flash of bright light i.e.; Cherenkov light and this phenomenon is known as the Cherenkov effect. Common transparent media where this effect is observed are air and water. To exhibit this effect and to travel faster than light in water i.e., more than 200,000 km/sec, a charged particle needs energy above 175 keV. Radioactive beta electrons often exhibit this effect while it is not possible for the slow and heavy alpha particles. In the air, for a small flash of light, the energy needed by Cherenkov light from the particles is greater than 21 MeV. This can never be fulfilled by radioactive electrons in the air.
Uses of Cherenkov Radiations
Studying Cosmic Showers in astrophysics: Observations made in astrophysics have shown that the properties of astronomical objects with high-frequency gamma rays can be determined using the Cherenkov radiations and cosmic showers in space can be detected.
Imaging of Radioactive Isotopes in the Medicine field: Recently, the Cherenkov light has been used to produce images of various substances in the body. This attempt was aimed at imaging for diagnostic value demonstration and the radioactive elements used were fluorine (13), nitrogen (13), iodine (131), phosphorus (32), and yttrium (90).
For Detecting Labelled Biomolecules: Using Cherenkov radiation, selective biological molecules of low concentrations can be detected. On introducing radioactive elements such as P-32 i.e., Phosphorous 32 by synthetic and enzymatic procedures, the dissociation rates and affinity constants are determined in the biomolecules.
Identification of Nature of Particles in High Energy Experiments: For the detection of high energy charged particles, such as beta particles, in nuclear fission decay, Cherenkov radiation is used. The characteristics of the blue light emitted from the fuel rods; it is also used for verifying the presence of nuclear fuel spent in pools.
Recommended Articles:
Characteristics of EM Waves
Characteristics of Sound Waves
Charge Transfer: Mechanism, Types, and Applications
Understanding of Charged Plane Sphere
Charging by Induction: Introduction, Importance, and Applications
No, the sky is blue due to the scattering of light by the atmosphere. It is not due to the Cerenkov effect. Imaging of Radioactive Isotopes in the Medicine field: Recently, the Cherenkov light has been used to produce images of various substances in the body. This attempt was aimed at imaging for diagnostic value demonstration and the radioactive elements used were fluorine (13), nitrogen (13), iodine (131), phosphorus (32), and yttrium (90). For Detecting Labelled Biomolecules: Using Cherenkov radiation, selective biological molecules of low concentrations can be detected. On introducing radioactive elements such as P-32 i.e., Phosphorous 32 by synthetic and enzymatic procedures, the dissociation rates and affinity constants are determined in the biomolecules. Soviet scientist Pavel Cherenkov was the first to detect Cherenkov radiations experimentally under the supervision of Sergey Vavilov at the Lebedev Institute in 1934. Yes, Observations made in astrophysics have shown that the properties of astronomical objects with high-frequency gamma rays can be determined using the Cherenkov radiations, and cosmic showers in space can be detected. Cherenkov Radiations FAQs
Are Cherenkov radiations responsible for the blue color of the sky?
State any two uses of Cherenkov radiations.
Who discovered the Cherenkov radiations?
Are Cherenkov radiations useful in studying cosmic showers in astrophysics?