In 1913, Danish physicist Niels Bohr introduced the atomic Hydrogen model and this was the first successful model to successfully explain the radiation spectra of atomic hydrogen. The Bohr atomic model theory made correct predictions for small-sized atoms like hydrogen, but poor spectral predictions for large atoms.
Failure of the Rutherford Model and the advent of the Bohr Atomic Model
The motion of the electrons around the nucleus in the Rutherford Atomic model was unstable because, according to classical mechanics and electromagnetic theory, any charged particle moving on a curved path emits electromagnetic radiation; thus, the electrons would lose their energy and spiral into the nucleus of the atom. To correct the problem, Bohr modified the Rutherford model by requiring that the electrons move in orbits of fixed size and energy in the atom. The energy of an electron depends on the size of the orbit and is lower for smaller orbits and higher for larger orbits. Radiation can occur only when the electron jumps from one orbit to another in the atom. The atom will be completely stable in the state with the smallest orbit since the electron cannot jump into the orbit of lower energy.
Planetary Model of the Atom
Neil Bohr, one of the founders of quantum mechanics, was interested in the much-debated topic of the time – the structure of the atom. There have been several theories that various scientists have postulated to determine the various atomic energies of the atom, from scientists like Thomson who described atoms as a giant pudding with raisins embedded in it to the Rutherford experiment that confirmed the presence of nuclei in the atom. But Bohr supported the planetary model, which asserted that electrons revolved around a positively charged nucleus in the atom just like the planets around the sun and when the electron is in one of these orbits, its energy is fixed.
Bohr’s explanation for Hydrogen Energy Levels
The Bohr Atomic model is used to describe the structure of hydrogen energy levels. The image below represents the shell structure, where each shell is associated with the principal quantum number that is represented by n. The number of energies at each level in the atom is expressed in work units, and thus the foremost energy is 13.598 work units of energy.
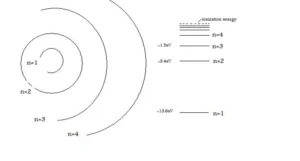
Absorption and Emission spectrum of the Hydrogen atom
According to Neil Bohr’s model, an electron would absorb energy in the form of photons to get excited to a higher level. After escaping to the higher energy level, which is also known as the excited state, the excited electron is less stable, and therefore, would rapidly emit a photon to come back to a lower i.e., stable energy level. The energy of the photon thus emitted is equal to the difference in energy between the two energy levels for a specific transition.
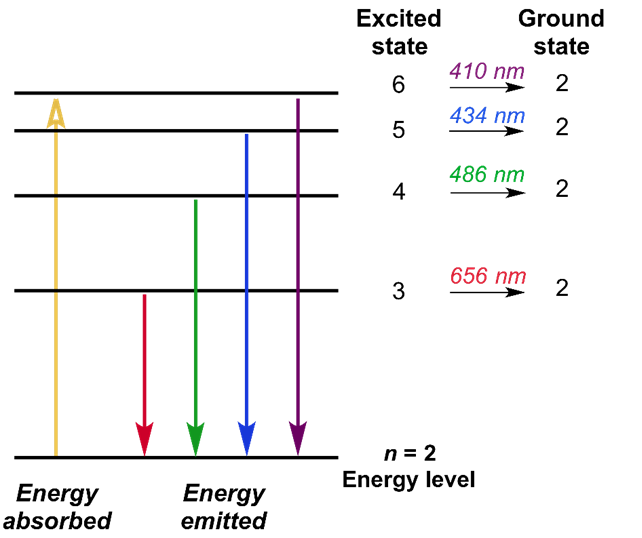
The movement of electrons between these energy levels produces a spectrum in the Hydrogen atom. Four different wavelengths of Hydrogen are described by the Balmer equation which is present in the visible light spectrum. These wavelengths are at 656nm, 486nm, 434nm, and 410nm. These wavelengths correspond to the emission of photons as an electron in an excited state transitions down to energy level n=2. The Rydberg formula generalizes the Balmer series for all energy level transitions. To get the Balmer lines, the Rydberg formula is used and this formula explains the different energies of transition that occur between energy levels. The Hydrogen atom can emit different wavelengths of light depending on the initial and final energy levels of the transition and it emits a photon with energy equal to the difference of the square of the final (nf) and initial (ni) energy levels.
Energy=R (1/n2f−1/n2i)
Let this be the first equation,
The energy of a photon is equal to hcλ
h is Planck’s constant =6.626*10-34m2kg/s,
c= speed of light in a vacuum and
λ= wavelength of emission.
E=hcλ
Let this be the second equation,
Combining the first and second equations we get the Rydberg Formula.
1/λ=R(1/n2f−1/n2i)
The Rydberg Constant, R= 10,973,731.6m−1 or 1.097×107m−1.
Drawbacks of Bohr’s Model of the hydrogen atom
Bohr’s model does not work well for complex atoms i.e., atoms that have more electrons. Some spectral lines split into multiple lines in the presence of a magnetic field i.e., the Zeeman effect and it could not explain why. Some spectral lines were more intense than others and it couldn’t explain why it was so. Also, Bohr’s idea of electrons existing in specific orbits with a known radius and velocity was contradicted by Heisenberg’s uncertainty principle.
Recommended Articles:
Black Body Radiation: Wien’s Displacement Law
Black Hole: Formation, Types, and Capacity
Blind Visually Impaired Braille
Blind Visually Impaired Non Optical Low Aids Vision
Blind Visually Impaired Optical Low Vision Aids
Bohr Model of the hydrogen atom was first proposed as the planetary model in 1913, but later an assumption concerning the electrons was made i.e., the quantization of the structure of atoms. Bohr proposed that electrons orbited the nucleus in an atom in specific orbits or shells with a fixed radius. The Bohr model of the atom was able to explain the Balmer series because of differences between the energy levels of the orbits matched the difference between energy levels of the line spectra. Zeeman effect is the splitting of a spectral line into two or more components of slightly different frequencies when the light source is placed in a magnetic field and Bohr’s model of the Hydrogen atom could not explain this effect. Bohr’s model does not work well for complex atoms i.e., atoms that have more electrons. Also, Bohr’s idea of electrons existing in specific orbits with a known radius and velocity was contradicted by Heisenberg’s uncertainty principle. Bohr Model of Hydrogen Atom FAQs
Explain Bohr’s theory for the Hydrogen atom.
How the Bohr model of the atom was able to explain the Balmer series?
What is the Zeeman effect?
Write any two drawbacks of Bohr’s Hydrogen atom model.