The Band Theory of Solids is a fundamental concept in solid-state physics that provides insight into the electronic properties of solids. Understanding the Band Theory is crucial for developing and advancing electronic devices and materials science. It explains how electrons in solids occupy specific energy levels and how the energy gap between these energy levels determines the electrical conductivity of a material.
In simple terms, a solid is composed of a large number of atoms, ions, or molecules arranged in a repeating pattern. The electrons of these atoms are not free to move around but are held in place by the strong bonds between the particles.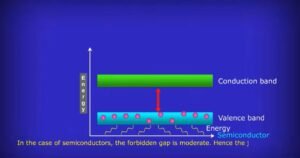
What are Energy Bands in Solids
Energy bands are specific energy levels occupied by electrons in solids. Electrons in solids occupy specific energy levels, known as energy bands. These energy bands can be visualized as a continuous energy spectrum, much like a series of steps. Energy bands in solids are a result of the interaction between the electrons of different atoms in the solid. This interaction causes the energy levels to become broadened and overlapping, forming the energy bands.
In solids, electrons occupy broadened and overlapping energy levels known as energy bands. These energy bands are the result of interactions between the electrons of different atoms in the solid. The energy band structure is a continuous spectrum that can be visualized as a series of steps.
Energy bands in solids-
There are three main types of energy bands in solids:
Valence band- The highest occupied energy band in a solid is called the valence band. The importance of the valence band is that electrons in the valence band are responsible for the chemical properties of a solid. The electrons are tightly bound to their atoms, as a result, cannot move electrons freely and making it difficult for the material to conduct electricity.
Conduction band- The lowest unoccupied energy band in a solid is called the conduction band. The importance of the conduction band is that electrons in the conduction band are free to move and contribute to the electrical conductivity of the material. Electrons in the conduction band are free to move, which makes it possible for a material to conduct electricity. The electrical conductivity of a solid is directly related to the availability of free electrons that conduct electricity in the conduction band.
Forbidden band- The energy gap between the valence and conduction bands is known as the forbidden band. The size of the forbidden band determines the electrical conductivity of a material and hence determines whether a material is an insulator, a metal, or a semiconductor.
If the forbidden band is small, electrons in the valence band can easily move to the conduction band, making the material a conductor. If the forbidden band is large, it is difficult for electrons to move from the valence band to the conduction band, making the material an insulator that results in lower conductivity. Semiconductors have a forbidden band of intermediate size that makes it possible to control the electrical conductivity of the material.
Energy band inside an atom
The energy levels are determined by the electron’s movement around the nucleus and their interactions with other electrons. However, in a solid, the interactions between the electrons of different atoms cause the energy levels to become broadened and overlap, forming the energy bands. So the energy levels inside an atom are well-defined and discrete.
The energy band in an atom refers to the range of energy levels that an electron can occupy within an atom. These energy levels are determined by the electron’s distance from the nucleus and its movement within the electron cloud. Electrons are arranged into shells and subshells, each with a distinct energy level, and can be thought of as existing in a band of energy.
Energy levels inside a solid made up of n-number of atoms
The energy levels inside a solid made up of an n-number of atoms form a continuous energy spectrum, forming the energy bands. The number of energy bands and their widths depend on the type and arrangement of the atoms in the solid.
The energy levels of individual atoms become closely packed and merge to form energy bands. The energy levels within a solid are influenced by the interactions between the atoms that make up the solid. In a crystal lattice, the electrons of adjacent atoms overlap, forming a continuous band of energy levels. The number and arrangement of the energy levels in a solid determine its electrical and thermal conductivity, as well as its optical properties.
Energy Band Structures in Different Systems
The energy levels of a material can vary depending on its states of matter such as solids, liquids, and gases. For example, in solids, energy levels are tightly packed and overlapping, while in liquids they are looser and more diffuse. Gases have the least organized energy levels, with electrons that are widely dispersed and have relatively large distances between energy levels. The energy levels within a material determine its physical and chemical properties, and changes in energy levels can result in changes in the material’s state of matter.
Applications and Importance
It is used to explain why some materials are insulators, why some are semiconductors, and why some conduct electricity. The theory has several uses in contemporary technology, including the creation of materials for catalysts, superconductors, and thermoelectric materials, as well as electrical devices including transistors, diodes, and solar cells. The capacity of band theory to anticipate and explain the behavior of materials allows for the creation of novel materials with certain features as well as the optimization of already existing materials for particular uses.
Recommended Articles:
Definition, Distance and Example of Average Velocity
Avogadro’s number: Definition, Law and Calculations
Azimuthal Quantum Number – History, Formula and Properties
What is Balanced and Unbalanced Force?
Balloon Experiments in Physics
Solid-state physics describes the electrical characteristics of solids in the band theory of solids. It sheds light on how the energy levels that electrons in solids occupy when they are present impact a material's ability to conduct electricity. The valence band refers to the highest occupied energy band in a solid. A solid's chemical characteristics are determined by its valence band electrons, which are securely linked to their atoms and prevent the substance from conducting electricity. Based on the band theory, solids are divided into conductors, insulators, and semiconductors. The size of the forbidden band is determined by the strength of the interactions between the electrons present in different atoms in the solid. Stronger interactions between the electrons result in a larger forbidden band and lower electrical conductivity. Metals have a small forbidden band, allowing electrons to move freely and contribute to high electrical conductivity. Non-metals have a larger forbidden band, resulting in lower electrical conductivity. Band Theory Of Solids FAQs
What is solid-state band theory?
What is the valence band?
Based on band theory, how are solids classified?
What determines the size of the forbidden band?
What is the difference between a metal and a non-metal based on the Band Theory of Solids?