The azimuthal quantum number is a set of non-negative integers that define the shape of an orbital in an atom. It is denoted by l where l = 0, 1, 2, 3, 4, and so on.
- It is also called the orbital angular momentum quantum number or orbital quantum number as it determines the orbital angular momentum, which is analogous to the classical angular momentum.
- The azimuthal quantum number is the second in a series of quantum numbers that describe the distinct quantum state of an electron in an atom (the others being the principal quantum number n, the magnetic quantum number m, and the spin quantum number).
Azimuthal Quantum Number
Azimuthal Quantum Number was put forward by Arnold Sommerfeld, a German theoretical physicist. Azimuthal Quantum Number is one of the four quantum numbers that locate an electron in an atom.
- The other quantum numbers are the principal quantum number, magnetic quantum number, and spin quantum number.
- This quantum number is important as it governs the geometry of an orbital and the orbital angular momentum.
History of Azimuthal Quantum Number
Azimuthal Quantum Number was carried over from Neil Bohr’s Model of the Atom and was introduced by Arnold Sommerfeld.
- The Bohr model was inspired by the spectroscopic analysis of the atom in combination with the Rutherford Atomic Model and the lowest quantum level was found to have an angular momentum of zero.
- Sommerfeld refined Neil Bohr’s semi-classical model by replacing circular orbits with elliptic ones.
- Spherical orbitals in the atom were similar (in the lowest-energy state) to a rope oscillating in a large “horizontal” circle.
The formula of Azimuthal Quantum Number
Arnold Sommerfeld introduced the azimuthal quantum number in his atomic structure. As a result, Arnold Sommerfeld calculates the angular momentum of an electron as it moves elliptically around the nucleus of an atom.
- As a result, the azimuthal or angular momentum quantum number determines the general geometric shapes of an electron cloud or orbitals in an atom.
For a given value of n, the possible values of l range from 0 to 1. (n-1).
As a result, the magnetic quantum numbers l= 0,1,2,3,4…. (n-1) are generated. However, the total number of different values of l equals n. l= 0 or 1s-orbital when n=1. However, 2p-orbitals for n=2, l= 0,1 or 2s.
- When n=3 and l=0, the orbitals are ls, 2s, or 3s, 3p, and 3d orbitals. l = 1 represents a p subshell then it has three dumbbell-shaped orbitals.
- If l = 2 represents a d subshell and is more complicated than p; it has 4 dumbbell-shaped orbitals and 1 doughnut-shaped orbital. If l = 3 is an f subshell, which is more complex than d and has 7 orbitals and as the value of the azimuthal quantum increases, the subshell becomes more convoluted.
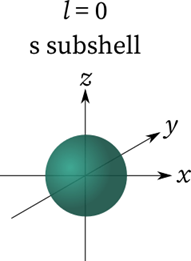
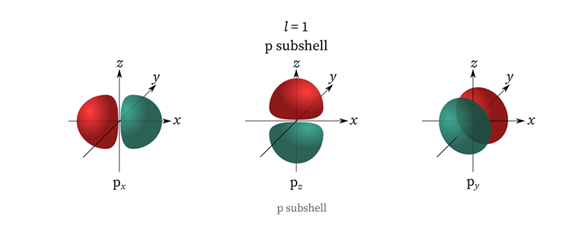
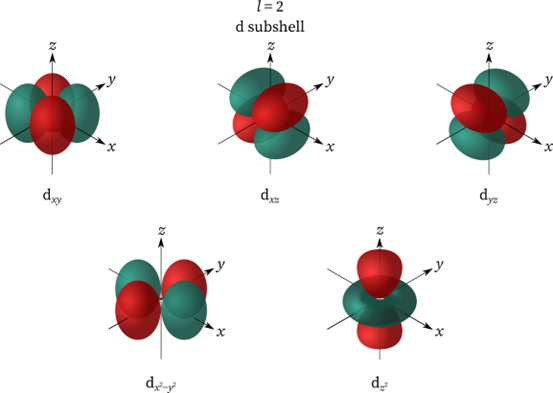
Properties of Azimuthal Quantum Number
- An s, p, d, and f subshell with a different form is designated by a value of the azimuthal quantum number, and the value of l, or the Azimuthal Quantum Number is determined by the value of a primary quantum number.
- The form of an orbital in an atom is determined by the azimuthal (orbital angular momentum) quantum number. Its value is always equal to the total number of angular nodes contained in the orbital and it is symbolized by the alphabet l.
- The orbital angular momentum quantum number also called the orbital quantum number or second quantum number is denoted by the letter l. This value controls the orbital angular momentum as well as the shape of the orbital and a p orbital coupled with an azimuthal quantum number of 1 is an example of the angular quantum momentum number.
- Apart from the main quantum number i.e., n, spectroscopic notation, the magnetic quantum number m, and spin quantum number s, the azimuthal quantum number l is another set of quantum numbers that define an electron’s unique quantum state. It is usually described as the quantum number attached to an atomic electron’s angular momentum.
Recommended Articles:
Atomic Radii: Introduction, States and Variation
Atomic Spectra – Absorption, States, Structures, and Applications
Average Speed and Average Velocity
Definition, Distance and Example of Average Velocity
Avogadro’s number: Definition, Law and Calculations
The subsidiary quantum number also called the azimuthal quantum number, is denoted by the letter l. It denotes which subshell the electron in an atom belongs to. It also determines the geometry of the electron’s occupied orbital and it is worth is determined by the value of the primary quantum number i.e., n. The azimuthal quantum number provides us with the following information: The number of subshells found in the primary shell of an atom. Subshell energies or relative energies. For the orbital 3p, 3 represents the principal quantum number i.e., n while p represents the azimuthal quantum number l. In integer form, subshell p equals 1 and has a dumbbell shape. Azimuthal quantum number describes the shape of the orbital and is denoted by l. Values of l are from zero to n-1. For the orbital 3d, 3 represents the principal quantum number i.e., n while d represents the azimuthal quantum number l. In integer form, subshell d equals 2 and has 4 dumbbell-shaped orbitals and 1 doughnut-shaped orbital. Azimuthal Quantum Number FAQs
What do you mean by subsidiary quantum number?
What is the significance of the Azimuthal Quantum number?
What is the Azimuthal quantum number of 3p?
What is the range of the Azimuthal quantum number?
What is the Azimuthal quantum number of 3d?
