If you want to learn about Avogadro’s number, you have come to the right place!
In this article, we will discuss Avogadro’s number. We will know what different physical and chemical aspects where Avogadro’s number plays an important role. After learning this we will learn the different applications of Avogadro’s number.
The purpose of this article is to understand Avogadro’s number and its characteristics. You will also find many other important information that you might need to know. If you do not have time to read all of the information, then I would suggest reading the introduction sections first.
Introduction
In science we have to deal with the fact that we can’t see molecules they’re just way too small. But they still obey stoichiometry that is they react in certain proportions. These proportions have nothing to do with mass. Since every molecule has a different mass rather they are by number as we saw in balancing equations. so in chemistry, we need a way to talk about molecules numerically and that number has to be so big that it represents a number of molecules that we can see with our eyes.
Definition of Avogadro’s number
Before defining Avogadro’s number we should understand Avogadro’s law. Avogadro’s law states that at normal pressure and temperature condition, an equal volume of different gases contains the same number of molecules whatever the substance is.
Avogadro’s number is the number of atoms or molecules present in one mole (mol) of substance. There are 6.022 x 1023 molecules present in every one mole of a substance. The unit of Avogadro number is mol-1.
For example, the molecular weight of one mole N2 is 28. So 28 mole of N2 contains 6.022 x 1023 molecules at NTP.
It is also observed from Avogadro’s rule that the volume occupied by one gram-mole of gas is approximately 22.4 liters at standard temperature and pressure (0 °C, 1 atmosphere).
Mathematical Representation of Avogadro’s law
It is also revealed from Avogadro’s law that the volume of any gas is directly proportional to the amount of gas substance. Let a gas having volume V and the amount of substance present in the gas is n. so,
Or,
Where k is a constant
Thus,
According to this the graph between the volume and number of particles should be a straight line passing through the origin.
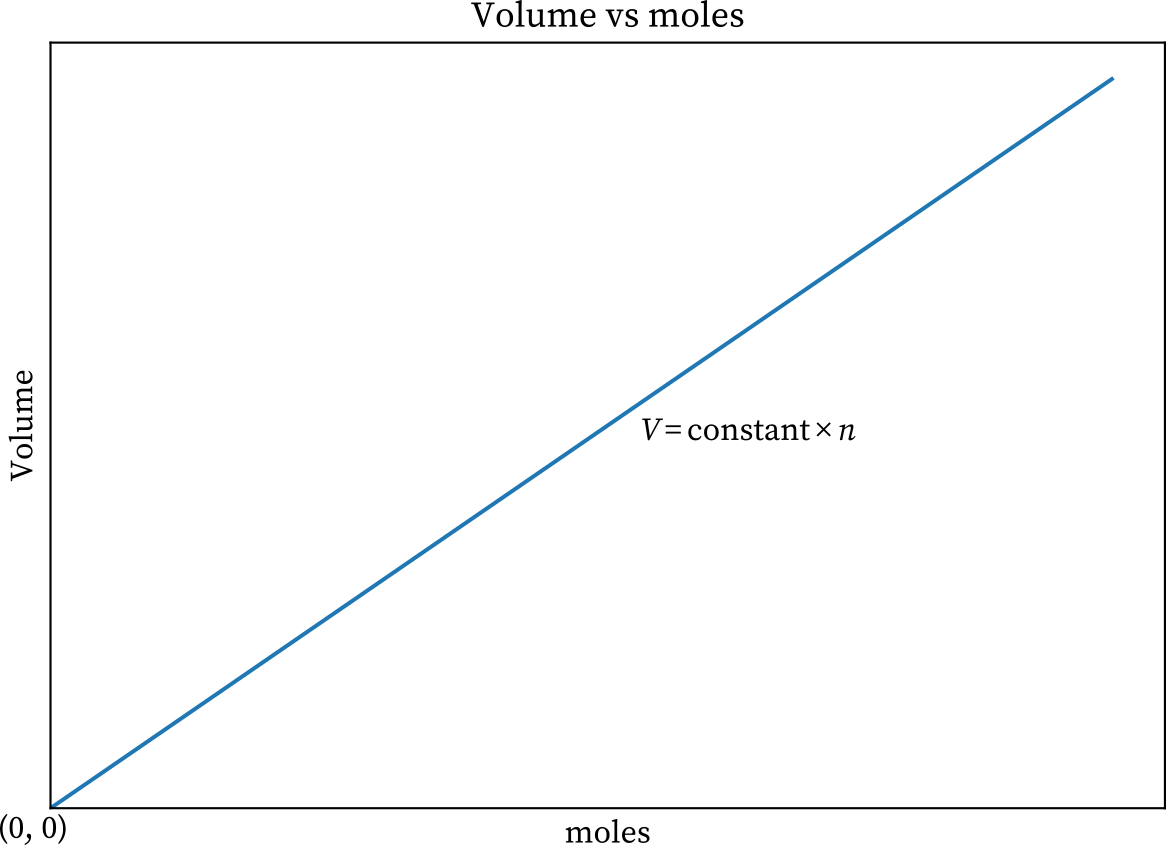
Fig- 1
Stoichiometric calculations
Avogadro’s number is the number of carbon atoms in exactly 12 grams. One carbon atom with six protons and six neutrons weighs 12 atomic mass units so a mole is the number of carbon atoms that weigh that same amount but in grams which is an amount that we can see and touch and do chemistry with. so the mole is the way to go between atomic mass units and grams.
let’s try one for practice now that we understand the mole we can do stoichiometric calculations which allow us to make predictions about a chemical reaction for example let’s say we are performing the following combustion reaction
we start with 20 grams of propane and we want to know what mass of water we are going to produce. this is very powerful because mathematical predictions like these are what enable us to use our understanding of Chemistry to create technology. so what we need to do is convert this mass of propane into moles because knowing the mass of something tells us nothing about the mass of something else we can expect since every compound has a different mass but these coefficients tell us about the numbers of molecules involved and moles are a number so let’s convert to moles. we can do this by using the molar mass of each substance. for propane that is 44.
Remember when we convert between units we multiplied by a fraction equal to 1. this many grams of propane does equal one mole which is why this doesn’t alter the quantity so we can see the twenty grams of propane is actually 0.45 moles of propane. That’s the number of molecules. If we have that we know how many moles of water to expect because there is a four-to-one ratio.
And now we know how many moles of water to anticipate, which is exactly four times that of moles of propane.
Recommended Articles:
Read all about Atomic Number Mass Number
Atomic Radii: Introduction, States and Variation
Atomic Spectra – Absorption, States, Structures, and Applications
Average Speed and Average Velocity
Definition, Distance and Example of Average Velocity
Avogadro’s number is the number of atoms or molecules present in one mole (mol) of substance. There are 6.022 x 1023 molecules present in every one mole of the substance. The unit of Avogadro number is mol-1. Avogadro’s law states that at normal pressure and temperature condition, equal volume of different gases contains the same number of molecules. One excellent illustration of Avogadro's law is the process of respiration. When people breathe in, their lungs' volume increases along with the amount of air they can hold in molar terms (expansion of the lungs). There are the following limitations of Avogadro law. Avogadro law is only described for real gases. Avogadro’s number FAQs
Explain Avogadro’s number.
What is Avogadro’s law?
Give a real-life example of Avogadro’s Law
What are the different limitations of Avogadro law.
It is not valid at low temperatures and high pressure as a real gas behaves like an ideal gas under that conditions.
It is also not applicable to heavy molecules.
