Introduction
Radiation is a form of energy produced when an unstable parent nucleus undergoes radioactive decay. Radiation travels a certain distance from the source in the form of excited waves/particles. alpha, beta and gamma particles; These particles have different properties and effects. The
alpha particles are named after the Greek letter “α”. The symbol for an alpha particle is α or α²⁺.
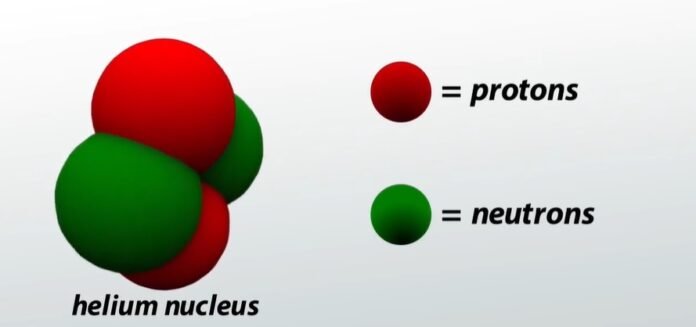
Because these particles coincide with helium nuclei. This is why an alpha particle can be written as He²⁺ or 24He, representing a helium ion with a charge of +2 (minus two electrons).
alpha particles are known as alpha rays or alpha radiation. This particle consists of two protons and two neutrons, forming a particle that is isotropic to the helium-4 nucleus. These particles are formed during α-decay.
Fundamental Properties of Alpha Particles
An alpha particle has twice the positive charge of a proton, equal to the charge of a helium nucleus. The mass of an α particle is 4 times the mass of a hydrogen atom, which is equal to the mass of a helium atom.
(The above two properties prove that an alpha particle is equal to a helium atom that has lost two of its orbital electrons or a double ionized helium atom.) The velocity of an alpha particle is 1.4 x 10⁷ m/ s to 2.1 x 10⁷ m/s range.
Due to the large mass of alpha particles, the penetrating power of these particles is small. The penetrating power is 1/10³ times that of beta rays and 1/10⁵ times that of gamma rays.
Alpha rays can be blocked by an aluminum plate with a thickness of 0.02 cm, despite their large size and low penetrating power.
Alpha particles have low penetrating power, but can burn the human body.
Because of their large mass and high velocity, these particles exhibit excessive ionization efficiency. This means that one alpha particle can create thousands of ions before being absorbed.
The distance an alpha particle travels in air depends on the radioactive source that produced it. At normal atmospheric pressure, alpha particles range from 3 to 8 cm.
Alpha particles produce fluorescence in some materials, such as barium cyanide and zinc sulfide (ZnS). Alpha particles partially affect the photo plate.
Alpha particles are deflected by both electric and magnetic fields at smaller angles. Alpha particles are distorted as they pass through the thin metal foil. Most of the particles were disordered at small angles. However, some of them are off at an angle greater than right angle.
Uses of Alpha Radiations
Alpha radiations are very popular in day-to-day applications. Some of its uses are:
For Treating Cancer Patients.
We use alpha particles in cancer treatment. When treating cancer patients, doctors use a technology called open source radiation therapy. This technique involves injecting small amounts of alpha particles, such as radium-226, into cancerous tumors. We use radium-223 to treat bone cancer.
Static Eliminator
Static neutralizers are common methods used in industries such as paper mills. This method helps to eliminate static electricity in industrial areas. In this method, the following happens: Alpha particles attract free electrons to themselves. thus reducing the possibility of static electricity.
Pacemaker Battery
Alpha radiation can be used as an energy source to extend battery life. Alpha radiation from plutonium-238 is the best fuel source for pacemakers.
Oil Industries
Alpha particles are the best source of energy, especially in remote areas. Strontium-90 alpha radiation is the best source for extending battery life..
Smoke Detectors
Smoke detectors typically fall into the following two categories:
· Photoelectric smoke detector
· Ionization smoke chamber detector.
Smoke chamber ionization detectors are common household items that keep us safe by alerting us to smoke in our homes. This detector uses a small amount of the isotope of americium, namely americium-241, as a source of alpha particles. Alpha radiation ionizes air molecules, causing a small current to flow between the electrodes.This current is amazing.
Recommended Articles:
Process, Occurrence, Measured of Alpha Decay
Air Composition Properties and Its Properties
Aerofoil: Introduction, Terminology, and Types
Advanced Sunrise And Delayed Sunset
Adiabatic Process: Definition, Process, Work, and Temperature
Alpha Particle Mass - Definition, Characteristics, Characteristics and Uses can be defined as a radioactive particle composed of two protons and two neutrons. It is nothing more than a helium nucleus, i.e. a helium atom without electrons. It is formed in the process of radioactive decay in which radioactive elements emit alpha particles. They are easily absorbed by cells. They pose a more intrinsic danger than the beta particles produced by beta decay and gamma particles. They can enter a person's internal organs through inhalation, ingestion, absorption, or injection. They can damage the sensitive living tissue present in our body. It can also damage DNA and cause cancer with prolonged exposure. Therefore, many precautions are taken wherever studies of interactions with alpha particles are conducted. Alpha particles are heavier than other particles associated with nuclear radiation. Because of this, the speed is relatively low. It moves at 5-7% of the speed of light, 2,000,000 m/s. This is the alpha particle's natural speed. Alpha particles can be dangerous in the following ways: Alpha particles help treat cancer. Proper care should be taken when introducing the alpha emitter through the incision. If these rays hit adjacent organs in the body, they could be damaged. Alpha radiation from nuclear power plants can penetrate DNA, damage cells, and cause cancer Alpha Particles Mass FAQs
What are Alpha Particle Mass – Definition, Characteristics, Properties and Uses?
What are the dangers associated with Alpha Particle Mass – Definition, Characteristics, Properties and Uses?
What is the speed of Alpha Particles?
How Alpha Particles Can be Dangerous?