Introduction
Work and energy are two of the most elementary yet important concepts in Physics. When a force is applied to an object which particularly causes its displacement, work is said to be done. Energy is the capacity to do the work.
What is Work?
Work is said to be done when a force that is applied makes it move through a distance. In layman’s language, even holding a heavy object is work. However, in the case of physics, it is not. When a person holds a heavy object, there is no particular force that is being applied to it.
The work done is only possible when a particular force acts on the object; it is equal to the magnitude of the force, which is then multiplied by the distance moved in the direction of the force.
The work is a scalar quantity as it has no direction and only magnitude.
The formula of Work Done
Work done refers to the product of the force multiplied by the direction of the displacement as well as the magnitude of the displacement.
W= F cos ϴ= Fd
Here
W= Work Done
F= The Force Applied
D= Displacement
ϴ= The angle between the force and the displacement
F cos ϴ= The component of the force which is in the direction of the displacement.
From the above equation, we can understand that if there is no displacement, work will not be done, despite the strength of the force.
No work is done when the displacement is zero, the force is zero, and the force, as well as displacement are perpendicular to each other.
SI Unit of Work
When work is said to be done, it is represented by the SI unit Joule which is J.
Mathematical Example of Work Done
Let us consider an object which is pulled across a surface by a force of 80 N and acting parallel to the surface. What is the amount of work that is done by the force that is moving the object at a distance of 10 m?
Now the question gives us
F= 80 N d= 10m.
Now, as the object is moving parallel, the ϴ =0.
So then
W= Fd cos ϴ
W= 80 x 10 x cos 0
W= 800 J as we know Cos 0=1
Energy
Energy is the potential ability in order to perform the work. It is important to understand that energy cannot be created or destroyed per se; it just gets transformed from one particular form to the other. The SI unit of work, as well as energy, is the same, and both of them are represented by Joules.
Essentially among the major types of energy, there are two divisions of energy. One being kinetic and the other being potential energy.
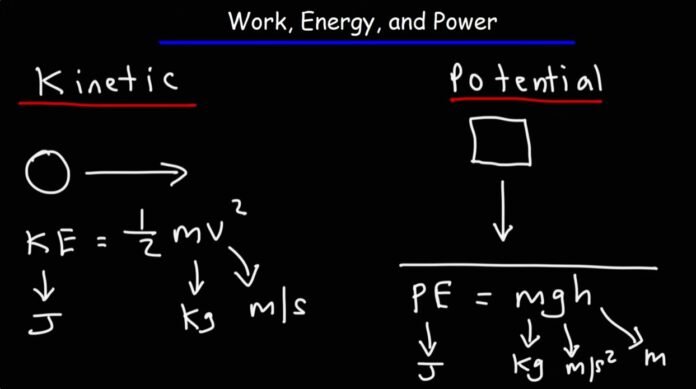
Kinetic Energy
Kinetic Energy refers to the energy of the object which is in motion. There is a theorem known as the work-energy theorem, which shows that the net work done is equal to the change in the kinetic energy.
W net = K. E. f − K. E. o
Potential Energy
Potential energy refers to stored energy. It is essentially the particular ability of a system to do work due to its structure present internally. The potential energy is essentially measured in joules.
Different Types of Energy
The following are some of the different types of energy.
Mechanical Wave Energy
A mechanical wave is a particular wave which is resulted from the oscillation of matter, thereby releasing energy through the medium. The amount of energy of the wave depends upon its amplitude as well as frequency.
Mechanical Energy
Mechanical energy is the energy of an object which is in motion or the energy which is stored in the objects due to their position.
Chemical Energy
Chemical energy is the energy of chemical substances, which is released when there is a chemical reaction and elements are transformed into other substances.
Electric Energy
Electric energy is an Energy that is related to the force of the electrically charged particle and majorly depends upon the movement of electrically charged particles.
Magnetic Energy
Magnetic energy is the movement of the charge of the electrons in the different particles. It is a movement that generates a current resulting in the electron behaving like a magnet.
Nuclear Energy
Nuclear Energy is the energy released from the nucleus, which is the core of atoms and is majorly made up of protons as well as neutrons.
Elastic Energy
Elastic energy is the energy that is stored by compressing or stretching an elastic object by a particular force, like that of stretching a spring.
Gravitational Energy
It is the potential energy an object has in relation to another massive object, essentially due to its gravity. So when two objects fall towards each other, the potential energy gets converted into kinetic energy.
Ionization Energy
This energy refers to the potential capability of an element to enter into a chemical reaction where ion formation and donation of electrons.
Elastic Energy
This is a type of energy that is stored in a particular object when there is a temporary strain on it. Here the energy is stored in the bonds between the atoms.
The Relation Between Work and Energy
Energy is essentially transferred to an object so as to move it. Essentially transferring energy can take the form of a force. The amount of energy that is transferred by force is known as work done. So we can say that there is a direct relationship between energy and work.
Work and energy are directly proportional to each other. This also takes us to the work energy theorem.
Work-Energy Theorem
The work-energy theorem, which is also known as the principle of work and kinetic energy, states that the total work done is essentially the summation of all force action on an object which is essentially equal to the change in its kinetic energy.
Recommended Articles:
Working Principle of an Electrical Fuse
Yield Strength
Youngs Modulus Elastic Modulus
Brief Introduction of Zener Diode
Important notes on Derivation of Bulk modulus