Some factors are responsible for the transfer of heat from one system to another such as, temperature , area of cross-section and some external environment. So the process in which there is no exchange of heat take place is known as an adiabatic process. In this let’s understand in detail the adiabatic process, example, adiabatic law, and its derivation.
Definition
The adiabatic process is a thermodynamics process in which there is no heat interaction during the process (Q = 0).
In another word, we can say that no supply and rejection of heat takes place during the compression and expansion process.
The system can be considered to be perfectly insulated. In this process, energy is transferred only as work. Any process that occurs inside a container that is a good thermal insulator is also adiabatic.
Adiabatic Process Examples
1. Hot tea leaped in the thermos.
2. A lightning strike
3. The Turbines -they utilize heat as a source to produce work
Law of Adiabatic process
P=Constant
Where,
- P is the pressure of the system.
- V is the volume of the system.
- y is the adiabatic constant
Also ,
y = Cp/Cv
- Cv is the heat capacity at a constant volume.
- Cp is the heat capacity at a constant pressure.
Derivation of Adiabatic Process.
From 1st Law of Thermodynamics, we can say that,
The change in internal energy of a system is equal to the heat added to the system excluding the work done by the system.
Q = W + dU
The First law makes use of the key concepts of heat, system work, and internal energy.
Let us consider,
- dv is a small increase in volume per unit mass.
- dq is a small quantity of heat per unit mass.
- dT is a small temperature rise.
Therefore, it can be written as,
dq = CVdT + pdv
By definition,
dq = 0
CV dT + pdv = 0
CVdT + pdv = 0 ————(1)
Also we have ,
pv = RT ————-(2)
Differentiating equation 2 , we get
pdv + vdp = RdT ————-(3)
Putting the value of dT from equation 3 in equation 1 we get,
+ pdv=0
Cv(pdv +vdp) +Rpdv=0 ————-(4)
Here ,
R = Cp- Cv ————-(5)
Putting equation 5 in equation 4 we get,
Cv(pdv +vdp) + (Cp- Cv)pdv = 0 ————-(6)
On solving equation 6 we get,
Cvpdv + vdp + pdv – pdv=0
vdp + pdv =0 ————-(7)
Dividing equation 7 with CVpv we get ,
+()=0 ————-(8)
Putting Cp/Cv=y in equation 8 we get,
+ y=0 where, y= ————-(9)
On integration equation 9 we get,
+ y =0 ————(10)
On solving equation 10 we get,
lop +lo = loC ————-(11)
From equation 11 we can say that,
P= Constant
This is called adiabatic process law.
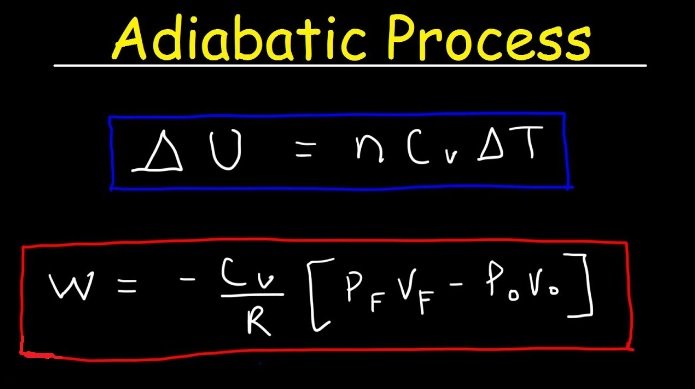
Work done in an adiabatic process.
As we know that the adiabatic process is a thermodynamics process in which there is no heat interaction during the process.
i.e. Q = 0
So it provides perfect insulation and an extremely fast process.
Also,
According to adiabatic process law,
P= Constant
When work is done by a thermodynamic system , it is usually a gas that is doing the work .
For non-constant pressure , the work can be imagined as the area under the pressure-volume curve which represents the process taking place. The more general expression for work done is given by :
Work done W=pdv
Or W=dv
W = kdv ————-(1)
As we know that, dx = applying this in equation we get,
W=k[
W=[]
W=[k]
Now, as we know that,
= = k
W=[]
W=[P2V2 – P1V1]
Finally, we get work done in an adiabatic process. Change in internal energy is positive so by this internal energy of the system would increase and hence the temperature of the gas increases.
Adiabatic relation between P and T
The Ideal gas equation for 1 mol is given by
V=
Here,
- V is the volume of the system.
- T is the temperature of the system.
- P is the pressure of the system.
Putting this in P = constant we get,
P[RT/P = constant
Adiabatic relation between V and T
The Ideal gas equation for 1 mol is given by
P=
Putting this in P = constant we get,
[RT/V] = constant
Important Points
1.In an adiabatic process the heat transfer is zero, so the entropy remains constant.
2. The Change in entropy in an adiabatic process is zero.
Recommended Articles:
Addition of Vectors and its Concept, Methods, and Examples
Actions of Transistor Based on Construction
Acoustics | Properties, Propagation, & Scale
Principle & Working Of Accelerometer
Accuracy and precision measurement
In the adiabatic process there is no heat interaction i.e. Q=0.So there is perfect insulation and the process is very fast. Adiabatic Process. When the tyre brust the temperature inside decreases. And the higher temperature outside the tyre transfer heat to it slowly not instantaneously. So there is no energy exchange takes place during the actual process. Basically, it is the ratio of heat capacity at constant pressure and constant volume. y = The Heat capacity ratio is also known as the adiabatic process. No heat exchange between the system and surroundings i.e. Q=0 No, it is a non-adiabatic process. When work is done adiabatically the change in internal energy is positive so that its internal process increases. Adiabatic Process FAQs
What is the Advantage of the adiabatic process?
When your bicycle tire suddenly bursts, which process is going?
What is the heat capacity ratio?
What is an adiabatic index?
Basically, it is the ratio of heat capacity at the constant pressure and constant volumeWhat is the condition for the adiabatic process?
Is a polytropic process an adiabatic process?
When work is done adiabatically its internal process increase or decreases?