What are Dielectrics?
Dielectrics, in general, can be described as materials that are very poor conductors of electric current. They are basically insulators and contain no free electron. Dielectrics can be easily polarized when an electric field is applied to it. Thus, their behaviour in an electric field is entirely different from that of conductors as would be clear from the following discussion.
Dielectric Materials
With respect to the atomic view, dielectric materials are classified into two categories.
Polar and Nonpolar Molecules helps us to understand the dielectric behavior in an electric field.
Polar Molecules
A polar molecule is one in which the ‘centres of gravity’ of the positive charges (i.e., protons) and negative charges (i.e., electrons) do not coincide. Such molecules are called permanent electric dipoles as these have permanent dipole moments. Some common polar molecules are HCl, H2O, N2O, NH3, H2S, C2H5OH, SO2.
In a molecule of HCl, there is an excess positive charge on the H-ion and an equal negative charge on the Cl-ion. The molecule, therefore, has a dipole moment at every instant and is a polar molecule. Another interesting example of polar molecules is H2O.
In the water molecule, two O-H bonds are not placed opposite to each other (unlike the CO2 molecule) but are inclined at an angle of about 105°. The hydrogen ion forms a dipole moment with each of the oxygen ion, and there is a net dipole moment
(i) In the absence of an electric field, the electric dipole moments of these polar molecules point in random directions [Fig. (b)] and cancel each other. Therefore, even though each molecule has a dipole moment, the average moment per unit volume is zero.
(ii) On the application of an electric field, the dipole moments of these molecules align themselves parallel, to the direction of the electric field as shown in figure (c). But this alignment is incomplete due to the thermal vibrations of the molecules. It is obvious that the alignment of the molecules with the applied field increases if:
- The electric intensity of the field is increased.
- Temperature is decreased.
It should be noted that increased electric intensity may also increase the dipole moment. It is due to the reason that with increased electric intensity, the distance between the centres of gravity of the positive and negative charges increases which results in an increase in the dipole moment.
Non-polar Molecules
A nonpolar molecule is one in which the centres of gravity of positive charges (i.e., protons) and negative charges (i.e., electrons) coincide. These molecules, thus, do not have any permanent dipole moment.
Some common examples of non-polar molecules are CO2, CCl4, oxygen (O2), nitrogen (N2), hydrogen (H2), methane (CH4) and ethane (C2H6).
In a molecule of CO2, the oxygen ions are symmetrically placed with respect to the carbon ion. Hence, the dipole moment is zero.
Dielectric Polarization
A dielectric may be made up of polar or nonpolar molecules. But the net effect of an external field is almost the same, i.e., the external field will compel the molecules to align their dipole moments along its own direction.
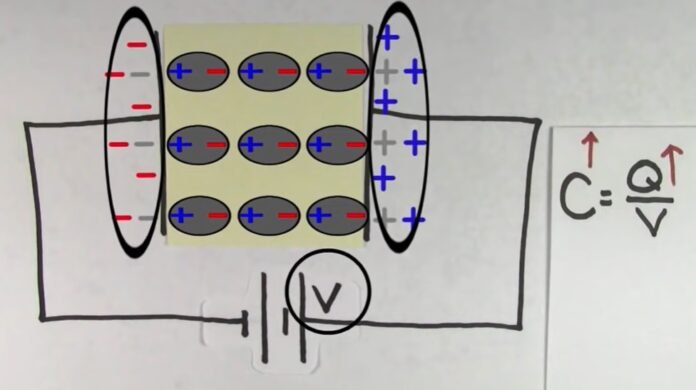
Let us consider a dielectric slab in an electric field which is acting in the direction shown in the figure. The arrangement of charges within the molecules of the dielectric in the electric field is as shown in the figure. The positive charges move in the direction of the field, and the negative charges in the opposite direction. In other words, the electric dipoles align themselves with the direction of the field. In this state, the entire dielectric and its molecules are said to be polarized.
The alignment of the dipole moments of the permanent or induced dipoles with the direction of the applied electric field is called polarization.
Within the two extremely thin surface layers indicated by shaded regions, there is an excess negative charge in one layer and an excess equal positive charge in the other layer.
The induced charges on the surfaces of the dielectric are due to these layers. These charges are not free but each is bound to a molecule lying in or near the surface. That is why these charges are called bound charges or fictitious charges. Within the remaining dielectric, the net charge per unit volume remains zero. Thus, although the dielectric is polarized, yet as a whole, it remains electrically neutral.
Obviously, the positive induced surface charge must be equal in magnitude to the negative induced surface charge. Thus, in polarization, the internal state of the slab is characterized not by an excess charge but by the relative displacement of the charges within it.
Polarization can thus also be thought of as a phenomenon in which an alignment of positive and negative charges takes place within the dielectric resulting in no net increase in the charge of the dielectric.
Meanwhile, polarization is defined as the electric dipole moment per unit volume.
The P vector has the same direction as the molecular dipole moment.
The SI unit of P is coulomb meter per cubic meter
or coulomb per square meter (C/m2).
The dimensional formula for P is:
Dipole moment/volume
=
- I = Current
- L = Length
- T = Time
What Is Dielectric Constant?
The dielectric constant of a substance can be defined as:
The ratio of the permittivity of the substance to the permittivity of the free space
It expresses the extent to which a material can hold electric flux in it.
Dielectric Constant Formula
It is mathematically expressed as:
Where,
- κ is the dielectric constant
- is the permittivity of the substance
- 0 is the permittivity of the free space
Dielectric Constant Units
As it is the ratio of two like entities, it is a unitless, dimensionless quantity.
Dielectric Constant Symbol
The relative permittivity of a dielectric substance is also called a Dielectric Constant, expressed using the Greek letter kappa ‘κ’.
Recommended Articles:
Diamagnetic Paramagnetic And Ferromagnetic
Diamagnetism: Introduction, Theory, Properties, Application And Comparison
Dichromate: Introduction, Structure, Preparation, Properties And Uses
Dielectric Constant: Introduction, Formula, Units, Symbol, And Theory
Dielectric Material And Dipole Moment
Dielectrics are insulators. They do not have any free electrons and hence do not conduct electricity. However, they transmit an electric field. Polar molecules and non-polar molecules. The molecules of a dielectric which act like tiny electric dipoles and possess a permanent dipole moment are called polar molecules. The molecules of a dielectric which do not possess a permanent dipole moment but get induced with a dipole moment in an electric field are called non-polar molecules. Dielectrics FAQs
What are dielectrics?
What are the two types of molecules of a dielectric?
What are polar molecules?
What are non-polar molecules?