Dichromate, also known as Cr2O72-, is a inorganic compound that is used as an oxidizing agent, and it has a wide range of applications. In this article, we will explore the structure, physical and chemical properties, and uses of dichromate.
Introduction
Dichloromethane is a widely used solvent in many industries, including pharmaceuticals, paint removers, metal cleaners, and pesticides. It is also used in the production of polycarbonates, acrylics, and other plastics. Despite its widespread use, dichloromethane is a hazardous substance that poses risks to human health and the environment.
What is Dichromate?
- Dichromate, also known as Cr2O72- , is a chemical compound that is composed of two chromium atoms and seven oxygen atoms.
- It has a molar mass of 215.988 g/mol, and it is a bright orange-red crystalline solid at room temperature.
- It is highly soluble in water, and a strong oxidizing agent .
Dichromate Structure
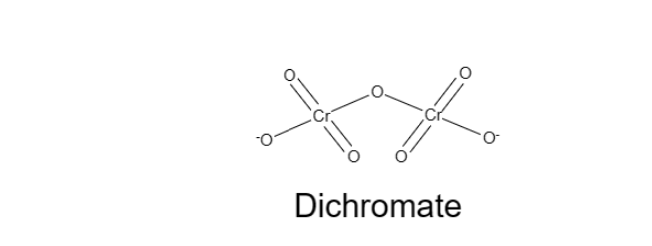
- It has a unique structure that is composed of two chromium atoms and seven oxygen atoms.
- The compound has a tetrahedral geometry, with two chromium atoms at the center of the tetrahedron and four oxygen atoms at the vertices. The remaining three oxygen atoms are located in a plane that is perpendicular to the tetrahedron.
- The structure of dichromate is highly symmetrical, and this symmetry is important in its reactivity and its applications as an oxidizing agent.
Preparation of dichromate
- One common way to prepare dichromate salts is by oxidizing a chromate salt (CrO4 2-) in acidic conditions. The balanced chemical equation for the preparation of potassium dichromate (K2Cr2O7) is:
![]()
- In this reaction, potassium chromate (K2CrO4) is mixed with sulfuric acid and oxygen gas in the presence of a catalyst such as manganese dioxide (MnO2).
- The mixture is heated and stirred vigorously, and the chromate is oxidized to dichromate.
- The byproducts of the reaction are potassium sulfate (K2SO4) and water (H2O).
- The potassium dichromate then be isolated by crystallization from the solution. This reaction is commonly used in the laboratory to prepare potassium dichromate for use as an oxidizing agent in various chemical reactions.
Physical Properties of Dichromate
- Appearance: It is a bright orange or red crystalline solid.
- Melting point: The melting point of dichromate is 357 °C.
- Boiling point: It decomposes before it boils.The decomposition temperature of dichromate is 196 °C
- Density: The density of dichromate is 2.676 g/cm3.
- Solubility: It is soluble in water and forms an acidic solution.
- Crystal structure: The crystal structure of dichromate is monoclinic.
- Hygroscopicity: It is hygroscopic, which means it can absorb moisture from the air.
Chemical Properties of Dichromate
Oxidizing Agent:
- Dichromate ion is a strong oxidizing agent. It can readily oxidize other substances, such as alcohols, aldehydes, and ketones.
- In the presence of an acid, dichromate ion can be reduced to Cr3+ ion, which itself is a powerful oxidizing agent.
Acidic Nature:
- Dichromate ion is an acidic compound. It can readily donate protons to other substances, making it a strong acid.
- When it reacts with a base, it forms the chromate ion (CrO42-) which is a weaker acid.
Precipitation Reaction:
- Dichromate ion form insoluble salts with certain cations, such as barium, lead, and silver ions.
- This property used in analytical chemistry for the identification and quantification of these cations in a sample. For example, a solution of barium chloride added to a solution containing dichromate ion will precipitate out barium dichromate, which has a characteristic yellow color.
Chemical Reaction of Dichromate Ion
Reduction:
- The reduction of the dichromate ion, involves the gain of electrons by the ion, resulting in the formation of a lower oxidation state of chromium.
- In this reaction, the dichromate ion is reduced to two chromium(III) ions (Cr3+) and water molecules (H2O) are formed.
- Reduction of dichromate by a reducing agent in balanced equations
![]()
Oxidation:
Oxidation of organic compounds by dichromate:
![]()
- This reaction shows dichromate used as an oxidizing agent to oxidize aldehydes to carboxylic acids. The reaction results in the formation of chromium(III) ions, carboxylic acids, and water.
Uses of Dichromate
- Oxidizing agent: Dichromate is a strong oxidizing agent and is used to oxidize alcohols to aldehydes and ketones in organic chemistry.
- Production of pigments: Dichromate is used to produce pigments like chrome yellow, which are used in paints and dyes.
- Electroplating: It is used as a component of the electrolyte in electroplating processes to deposit chromium on metals.
- Tanning of leather: It is used in the process of tanning leather to make it more durable and resistant to decay.
Recommended Articles:
Deuterium: Introduction, Properties, Uses, And Facts
Detergents And Surface Tension
Deuteron Mass: Introduction, Measured, Importance, Properties And Significance
Diamagnetic Paramagnetic And Ferromagnetic
Diamagnetism: Introduction, Theory, Properties, Application And Comparison
Dichromate is an inorganic compound composed of two chromium atoms and seven oxygen atoms. Its chemical formula is Cr2O72-. The compound has a highly symmetrical structure that is composed of two tetrahedral chromium centers that are bridged by oxygen atoms. Dichromate is an orange-red solid that is highly soluble in water. The compound has a melting point of 356 °C and a boiling point of 400 °C. Dichromate is a strong oxidizing agent and can easily oxidize a wide range of organic and inorganic compounds. Dichromate is used in various industrial processes, including the production of dyes, pigments, and chrome-plating solutions. Dichromate FAQs
What is dichromate and what is its structure?
What are the physical properties of dichromate?
What are the chemical properties of dichromate?
What are the uses of dichromate?
