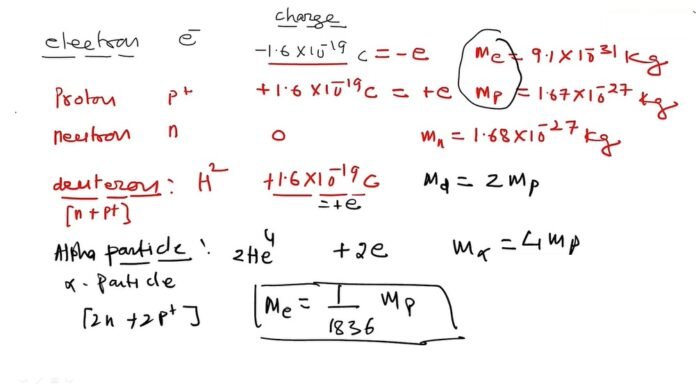Deuteron is the nucleus of deuterium, which is also known as heavy hydrogen. It is the simplest and the most common isotope of hydrogen. It has one proton and one neutron in its nucleus.
Introduction:
The deuteron mass is one of the most important properties of the deuteron. In this article, we will discuss the properties of the deuteron mass, how it is measured, and its significance in nuclear physics.
What is Deuteron Mass?
Deuteron mass is the mass of the nucleus of deuterium, which is the combination of one proton and one neutron. The mass of the deuteron is slightly larger than the sum of the masses of the proton and neutron, due to the binding energy that holds the nucleus together. This binding energy is the result of the strong nuclear force that acts between the protons and neutrons in the nucleus.
How is Deuteron Mass Measured?
The deuteron mass is measured using various experimental techniques. One of the most common techniques is mass spectroscopy, which involves ionizing the deuteron and then measuring its acceleration in an electric field. The mass of the deuteron can be determined by the rate of acceleration.
Importance of Studying Deuteron Mass
The study of the deuteron mass is important in understanding the properties of the nucleus of deuterium, which is the simplest nucleus with a neutron-proton pair.
- One of the most important properties of the deuteron mass is its binding energy, which is the energy required to separate the neutron and proton in the deuteron nucleus. The binding energy is related to the stability of the deuteron and its interactions with other particles. Accurate measurements of the deuteron mass and binding energy are crucial for understanding the properties of the deuteron and its role in nuclear physics.
The properties of the deuteron mass, such as its spin, electric quadrupole moment, and magnetic moment, are important in understanding the structure of the deuteron and its interactions with other particles. For example, the magnetic moment of the deuteron is used to study the structure of the deuteron and its interactions with external magnetic fields.
Furthermore, the deuteron is a potential fuel for fusion reactors, which have the potential to provide a clean and sustainable source of energy. The study of the deuteron mass is important in understanding the behavior of the deuteron in fusion reactions and in designing fusion reactors.
Properties of Deuteron Mass:
- Mass of Deuteron:
The mass of the deuteron is approximately 2.01410178 atomic mass units (amu). This value is slightly larger than the sum of the masses of a proton and neutron, which is approximately 1.008 amu.
- Binding Energy:
The binding energy of the deuteron is the energy that holds the proton and neutron together in the nucleus. The binding energy of the deuteron is approximately 2.22 MeV (mega electron volts).
- Spin:
The deuteron has a spin of 1, which means that it has a magnetic moment. This magnetic moment can be used to study the properties of the deuteron and the interactions between the deuteron and other particles.
- Electric Quadrupole Moment:
The deuteron has a non-zero electric quadrupole moment, which means that it has a non-spherical shape. This property is important in studying the structure of the deuteron and the interactions between the deuteron and other particles.
- Magnetic Moment:
The deuteron has a magnetic moment due to the spin of the proton and neutron in its nucleus. The magnetic moment of the deuteron is a crucial property for understanding the interactions between the deuteron and other particles.
Isospin:
Significance of Deuteron Mass:
- The deuteron mass is an important property of the nucleus of deuterium. It plays a crucial role in understanding the properties of the deuteron and the interactions between the deuteron and other particles. The deuteron mass is also used in various experimental techniques, such as mass spectroscopy and nuclear reactions.
- Another important property of the deuteron mass is its binding energy. The binding energy of the deuteron is the energy that holds the proton and neutron together in the nucleus. Without this binding energy, the nucleus would fall apart. The binding energy of the deuteron is also an important factor in understanding the stability of other nuclei.
- The magnetic moment of the deuteron is also important in understanding the interactions between the deuteron and other particles. It can be used to study the magnetic properties of the deuteron and how it interacts with external magnetic fields.
- The isospin of the deuteron is another important property. Isospin is a quantum number that characterizes the strong nuclear force between nucleons. It is important in understanding the properties of the deuteron and the interactions between the deuteron and other particles. It is also important in the study of the strong nuclear force, which is responsible for holding the nucleus together.
- The deuteron mass is used in various experimental techniques, such as mass spectroscopy and nuclear reactions. These techniques are used to study the properties of the deuteron and its interactions with other particles. Mass spectroscopy is used to measure the mass of the deuteron, while nuclear reactions can be used to determine its properties such as its binding energy, magnetic moment, and electric quadrupole moment.
It is also an important ingredient in nuclear fusion reactions, which are being studied as a potential source of clean energy.
In summary, the deuteron mass is an important property of the nucleus of deuterium.
Experimental Techniques Involving Deuteron Mass Mass Spectroscopy Nuclear Reactions
Experimental techniques involving the deuteron mass are crucial for understanding the properties of the deuteron and its interactions with other particles. Two important experimental techniques are mass spectroscopy and nuclear reactions.
- Mass Spectroscopy:
Mass spectroscopy is a powerful analytical technique used to measure the masses of atomic and molecular ions. It is based on the principle that ions can be separated according to their mass-to-charge ratio using electric and magnetic fields. In the case of the deuteron, mass spectroscopy is used to measure its mass accurately.
- Nuclear Reactions:
Nuclear reactions involving the deuteron are another important experimental technique used to study its properties. In nuclear reactions, a deuteron is accelerated and collided with another nucleus, producing various reaction products. The properties of these reaction products are then analyzed to gain information about the deuteron and the nucleus it collided with.
Nuclear reactions involving the deuteron are used in various fields, such as nuclear physics, astrophysics, and nuclear medicine. They are important in studying the properties of the deuteron, such as its binding energy, magnetic moment, and isospin. They are also used to study the properties of other nuclei and their interactions with the deuteron.
One example of a nuclear reaction involving the deuteron is the deuteron-induced reaction. In this reaction, a deuteron is collided with a target nucleus, producing various reaction products.
In summary, mass spectroscopy and nuclear reactions are important experimental techniques used to study the properties of the deuteron. Mass spectroscopy is used to accurately measure the mass of the deuteron, while nuclear reactions are used to study its properties and interactions with other particles. These experimental techniques are crucial in understanding the structure of the deuteron and its role in nuclear physics.
Recommended Articles:
Determination Of Focal Length Of Concave Mirror And Convex Lens
Determine Mass Of Two Different Objects By Using A Beam Balance
Determine Resistance Plotting Graph Potential Difference Versus Current
Deuterium: Introduction, Properties, Uses, And Facts
Detergents And Surface Tension
The mass of the deuteron is slightly larger than the sum of the masses of the proton and neutron. This is because of the binding energy that holds the proton and neutron together in the nucleus. The binding energy of the deuteron is important because it is the energy in the nucleus. Without this binding energy, the nucleus would fall apart. The binding energy of the deuteron is also an important factor in understanding the stability of other nuclei. The magnetic moment of the deuteron is important in understanding the interactions between the deuteron and other particles. It can be used to study the magnetic properties of the deuteron and how it interacts with external magnetic fields. The isospin of the deuteron is important in understanding the properties of the deuteron and the interactions between the deuteron and other particles. It is also important in the study of the strong nuclear force, which is responsible for holding the nucleus together. The deuteron mass is used in various experimental techniques, such as mass spectroscopy and nuclear reactions. These techniques are used to study the properties of the deuteron and its interactions with other particles. Mass spectroscopy is used to measure the mass of the deuteron, while nuclear reactions can be used to determine its properties such as its binding energy, magnetic moment, and electric quadrupole moment. Deuteron Mass FAQs
What is the difference between the mass of the deuteron and the sum of the masses of the proton and neutron?
Why is the binding energy of the deuteron important?
How is the magnetic moment of the deuteron important?
What is the significance of the isospin of the deuteron?
How is the deuteron mass used in experimental techniques?