The Davisson—Germer experiment which was conducted in the year 1927 first time measured the wavelengths of electrons. In the year 1937, C. J. Davisson shared the Nobel Prize in Physics with George Thomson who discovered experimental evidence of electron diffraction independently. According to the Copenhagen Interpretation of Quantum Mechanics, the wave-particle duality causes particles to display wave-like properties such as interference and spatial extension.
History of Davisson Germer Experiment
- Germer and Clinton J. Davisson studied the reflection of electron beams on the surface of nickel crystals.
- When the beam hits the crystal, the electrons are scattered in all directions by the nickel atoms and the intensity of the scattered electrons was measured in relation to the incident electron beam by their detector.
- The dispersed electrons in their normal polycrystalline samples were distributed in a very smooth angular distribution.
- When one of their samples was accidentally recrystallized in a laboratory mishap in the year 1925 resulting in a practically monocrystalline structure, strong peaks appeared in the angular distribution at angles.
- Other monocrystalline materials, as Davisson and Germer soon discovered, exhibited similar aberrant patterns, which differed depending on the angle of incidence, chemical composition, and orientation of the sample.
- When Davisson visited a meeting of the British Association for the Advancement of Science in Oxford in the year 1926, they finally realized what was going on.
- During the occasion, Born discussed de Broglie’s matter waves and Schrodinger’s wave mechanics and the quantum mechanical predictions for electron wavelength as a function of momentum p = h/p were perfectly validated by their subsequent measurements.
- However, unlike Thomson, their initial tests were carried out in the context of practical materials research on vacuum tube filaments, rather than under any specific theoretical guidance.
Set up of Davisson Germer Experiment
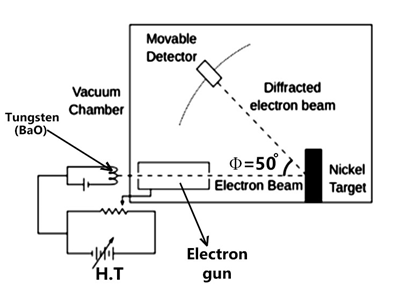
An electron gun comprising a tungsten filament F was coated with BaO i.e., barium oxide, and heated through a low-voltage power supply.
After applying suitable potential difference from a high voltage power supply, the electron gun emits electrons that were accelerated to a particular velocity.
These emitted electrons were made to pass through a cylinder perforated with fine holes along its axis, thus producing a finely collimated beam.
The beam produced from the cylinder is again made to fall on the surface of a nickel crystal and due to this, the electrons scatter in various directions.
The beam of electrons produced has a certain amount of intensity which is measured by the electron detector and after it is connected to a sensitive galvanometer, it is then moved on a circular scale to record the current.
By moving the detector on the circular scale at different positions that are changing the θ which is the angle between the incident and the scattered electron beams, the intensity of the scattered electron beam is measured for different values of the θ.
Observations of Davisson Germer Experiment
- By changing the angle of scattering, represented by θ, we can get the variation of intensity I of the scattered electrons.
- The accelerated voltage can change from 45 V to 68 V by varying the accelerating potential difference.
- A strong peak in the intensity of electrons can be observed with an accelerating voltage of 55 V and a scattering angle θ = 50º of an electron.
- This peak in intensity was due to the constructive interference of the electrons that are scattered from different layers of the regularly spaced atoms of the crystals.
- The wavelength of the waves was determined as 0.165 nanometers with the help of electron diffraction.
- Another observation that we can find here explains how the intensity of a scattered electron is never continuous and we can find both minimum and maximum values analogous to the maximum and minimum diffraction pattern gained via X-rays.
Relation between Davisson Germer Experiment and de Broglie Equation
According to the de Broglie equation,
λ=h/p
=1.227/ nm
λ=1.227/ nm
0.167 nm
Where λ is the wavelength associated with the electrons.
Therefore, the de Broglie relation and the wave nature of electrons were confirmed by the Davisson Germer Experiment.
Recommended Articles:
Cyclic Process: Introduction, Types, And FAQ
D’alembert’s Principle: Introduction, Derivation, Examples, And Applications
Darcy Weisbach Equation Derivation
DARCY’S LAW: Introduction, Equation, Application, Properties, And Limitation
The Dark side of the moon
An electron gun is a tungsten filament that emits electrons through thermionic emission when heated above a specific temperature. The Davisson Germer Experiment illustrated the wave nature of electrons, in continuation of the theorem of De-Broglie and gave confirmation to wave-particle quality. It is an apparatus built up for the purpose of measuring the energies of electrons scattered from the metal surface. To avoid electron deflection and scattering by the medium, a vacuum chamber is used in the Davisson Germer Experiment. According to the conclusion obtained by the Davisson-Germer experiment it was shown that electrons exhibit wave nature too and this conclusion supports the hypothesis given by De-Broglie regarding wave-particle duality of matter. Davisson Germer Experiment FAQs
What is an Electric Gun?
What is the importance of the Davisson Germer Experiment?
Why a vacuum chamber is used in the Davisson Germer Experiment?
What is the conclusion of the Davisson-Germer experiment?