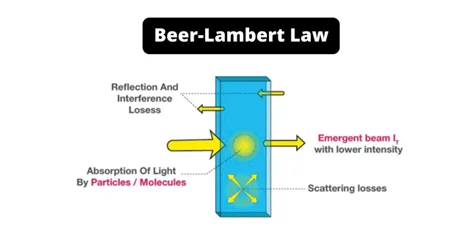
As formulated by German mathematician and chemist August Beer in 1852, Beer-Lambert Law states that for a given solution path length and concentration of the solution are directly proportional to the absorbance of the light.
- When monochrome light passes through a homogeneous solution, the intensity of the transmitted radiation drops at a constant rate as the thickness of the solution grows, and the solution concentration varies directly with the intensity of radiation received, according to Beer-Lambert.
Beer-Lambert Law
The Beer-Lambert law also known as Beer’s law, the Lambert–Beer law, or the Beer–Lambert–Bouguer law states that there is a linear relationship between the absorbance and the concentration of the solution that enables the concentration of a solution to be calculated by measuring its absorbance.
- This law relates the attenuation of light to the properties of the medium through which the light is traveling.
History of Beer-Lambert Law
This law was discovered in 1729 by Pierre Bouguer. Later, in the year 1760, Johann Lambert quoted Bouger’s discovery saying that the absorbance of a solution is directly proportional to the path length of light.
- Although Lambert didn’t claim this discovery, he was often credited with it. In 1852, August Beer discovered a related law that stated that the absorbance is proportional to the concentration of the given solution.
Beer-Lambert Law Equation
The Beer-Lambert law equation is given as follows
I = Ioe-μ(x)
Where,
I = Intensity,
Io = Initial Intensity,
μ = Coefficient of absorption,
x = Depth (meter).
Beer-Lambert Law Derivation
When the light rays travel through a solution, the quantity of transmitted or absorbed light is an exponential function of the molecular concentration of the solute i.e., the substance present in the smaller quantity and a function of the length of the path of radiation through the given solution.
Therefore, Beer-Lambert law derivation can be derived by the steps given below:
log I₀/I = εcl
Where,
I₀= the intensity of the incident light,
I= the intensity of light transmitted through the solution,
ε= the absorptivity or molar attenuation coefficient,
c= the concentration of the solute in mol l-1, and
L= the path length of the solution in centimetres
The above ratio i.e., I/I₀ is known as transmittance and absorbance is the logarithm of the inverse ratio i.e., I₀/I.
-Log I /I₀ = – log T = ε*c* L
Log I₀/I = A = ε* c* L
Therefore, the equation of Beer-Lambert law is A = εLc
The relationship between the amount of light transmitted to the detector after passing through the solution and the initial amount of light is known as transmittance.
ε = A/Lc
Therefore, A = log10Io/IT
Conditions for validation of Beer-Lambert Law
- The wave properties of the light used should be negligible, so it does not vary the attenuation in the medium.
- The attenuators must be able to function independently of each another.
- In the interaction volume, the attenuating medium must be a homogeneous medium where the light interacts with the solution for obvious reasons because any variation in the sample will affect the attenuation.
- Unless this is compensated for as in DOAS, the attenuating homogenous medium must not scatter the radiation—no turbidity.
- The incident rays must be in the form of parallel beams that travel the same distance through the absorbing medium.
- The incident rays should ideally be monochromatic, or at the very least have a narrower breadth than the attenuating transition. Otherwise, instead of a photodiode that cannot distinguish between different wavelengths, a spectrometer as a power detector is required.
Applications of Beer-Lambert Law
- This law is used to measure the concentration of various solutions, analyze oxidation, and measure polymer degradation. It also describes the attenuation of radiation through the atmosphere of the earth.
- This law can analyze biological substances that may contain organic or inorganic elements.
- The purity of any substance can be checked by measuring the absorbance of a compound using a spectrophotometer.
- By analyzing the absorption spectra of various chemicals in cell structures, this law can help determine the concentration.
- The principle of the Ultraviolet-Visible spectrometer is based on the Beer-Lambert law.
Limitations of Beer-Lambert Law
- This law is applicable under a low concentration range where interactions between molecules are not considered and not applicable under higher concentration as the molecules of the analyte have stronger intermolecular and electrostatic interactions due to the small amount of space between them.
- High concentrations alter not just the molar absorptivity of the solution but also the refractive index of the solution, resulting in deviations from the Beer-Lambert law.
- This law is only valid in monochromatic light.
Recommended Articles:
Barometer: Definition, Working, Pressure, Types and Unit
Basic Laws of Physics: Motion, Conservation of Energy, Mass and Thermodynamics
Basic Properties of Electrical Charge
Batteries In Series Parallel
Definition of Beats: Occur, Frequency, Formula, Effect and Applications
When different types of molecules are in equilibrium with each other and when fluorescent compounds are used. Beer-Lambert law fails at higher concentrations as the linearity of the law is limited to chemical and instrumental factors and when the solution has higher concentrations, the proximity between the molecules of the medium is so close that there are deviations in the absorptivity. Also, when the concentration is high, the refractive index of the medium changes. This law is applicable under a low concentration range where interactions between molecules are not considered and not applicable under higher concentration as the molecules of the analyte have stronger intermolecular and electrostatic interactions due to the small amount of space between them. This law is only valid in monochromatic light. Yes, a colorimeter is used to measure the concentration of a known solute in a given solution. Lambert’s law asserts that absorbance and path length of the sample is exactly related. Beer law asserts that the concentration and absorbance of the solution are exactly proportional to one other. Beer-Lambert Law FAQs
State any two conditions when Beer-Lambert Law is not obeyed.
Why Beer-Lambert Law fails when the concentration is higher?
State any two limitations of Beer-Lambart’s law.
Is Colorimeter used in the verification of the Beer-Lambert law?
State Lambert and Beer’s Law?