If you want to learn about Atomic radii, you have come to the right place!
In this article we will discuss about the atomic radii. We will know that what different physical and chemical aspects where atomic radii play an important role. After learning this we will see the periodic trends of atomic radii.
The purpose of this article is to understand the atomic radii and its characteristics. You will also find many other important information that you might need to know. If you do not have time to read all of the information, then I would suggest reading the introduction and conclusion sections first.
Introduction
As we know a atoms is a combination of electron, proton and neutrons. Protons and neutrons are responsible to make nucleus of the atoms whereas electron revolves and rotates around the nucleus in different orbits. Basically, an atomic radius is the distance between electrons present at outermost shell to the nucleus. But, as we know from the Heisenberg’s uncertainty principle that electron behave like a wave and exact position of electron cannot be find out. This principal is become very significant when we enter in microscopic world and consider electron proton and neutron in our study. Thus the boundary of the atoms is not defined. So, now it is difficult to define the atomics radii in terms of outermost shell.
So, the atomic radius is not measured directly for the individual atoms infect it is measured in bonded state or interaction states.
Bonded states
There are following ways to measure the atomic radius in bonded state.
- Covalent radius
- Van-dar wall radius
- Metallic radius
Covalent radius
To understand the covalent radius ones need to understand the covalent bond first. Covalent bond is formed by the sharing of electron clouds. From figure 1 let there is sharing of electron clouds take place between two similar atoms A and A.
A A 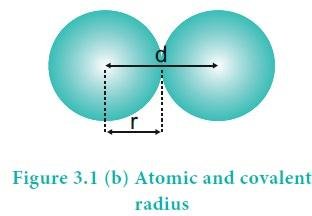
Fig-1
Then, the half of the distance between the centers of A and A is termed as covalent radius or covalent radii. In the figure covalent radius is represented by ‘r’. Also, the distance ‘d’ shown in the figure is known as bond length. So we can say that covalent radius is the half of bond length.
i.e.
If the atoms are not similar:
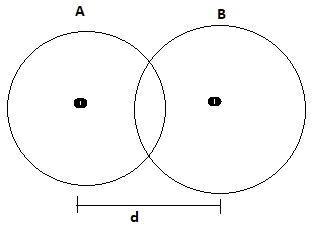
Fig-2
Let atoms A and B formed covalent bond then two conditions arise:
Case -1: Electro negativity of the atoms is same
If electro negativity of both of the atoms are same then the electron clouds distribute uniformly and the bond length is the sum of radius of both atoms and covalent radius is the half of bond length.
Case-2: Electro negativity of the atoms is not same
If electro negativity of both of the atoms is not same then, the electron clouds shift towards the atom having higher electro negativity. Let the electro negativity of atom A is higher than atom B. in this case atom A attracts the electron clouds of atom B.
The formula to obtain bond length in this condition is following
Where is the difference of electro negativity of atom A and B.
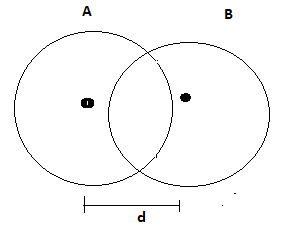
Fig-3
It is also to be noted that –) term shows that electron clouds of B move towards of A so the total distance between the center of A and B also reduced.
Van-dar wall radius
The molecules interact to each other to form a material. This force of attraction is also called Van-dar wall force. In this case two molecules are bonded together with intermolecular attraction not by any of the bond.
Let us consider two molecules of hydrogen. Here these molecules attract each other with intermolecular attraction and came closest to each other. dH2H2 is the minimum distance between these molecules shown in the figure. It is called bond length.
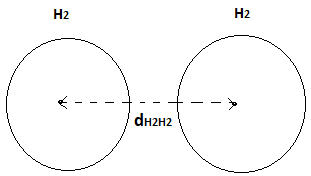
Fig-4
Now,
Where, is van-dar wall radius.
- The importance of van-der wall radius is to calculate the radius of inert gases. Because these gases not make any bond between similar atoms.
- van-der wall radius is always greater than covalent radius because in case of covalent radius the overlapping of atoms is taken which leads to reduce the distance between the atoms but in case of van-der wall radius there is no overlapping.
Metallic radius
Metallic radius is considered where metallic bonds are formed. Metallic bond is the type of bond in which metals electron attracts the nucleus of neighbor atoms. It is half of the distance between the centers of two bonded metal atoms.
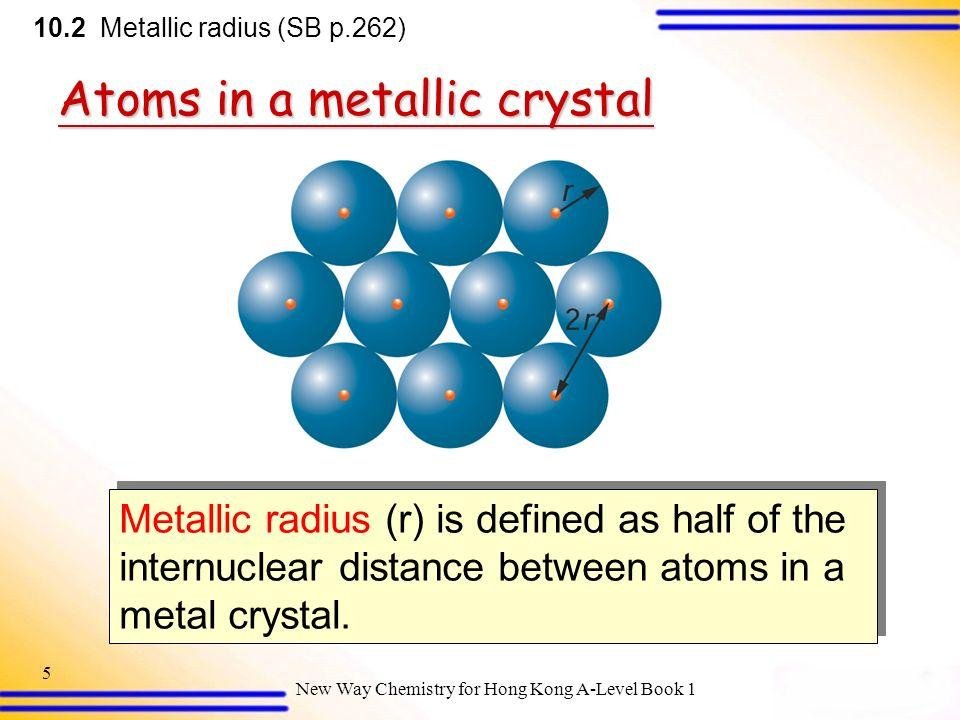
Fig-5
A metallic lattice is shown in the figure. Where, r is termed as metallic radius.
Metallic radius is greater than covalent radius but less than van-dar wall radius.
Covalent radius < Metallic radius < Van-dar wall radius
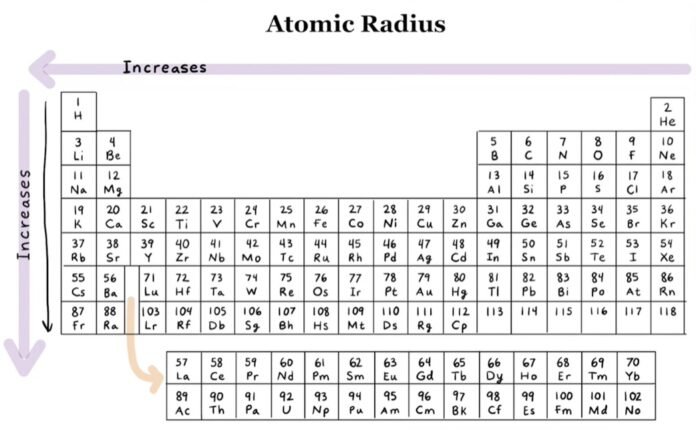
Variation of atomic radii in periodic table
Along the Period
When we move left to right in periodic table the atomic number is increased which means the number of protons in the nucleolus is increased. Whereas the number of orbits is remain same. It can be observed that the strength of force of attraction between electron and proton depends upon the number of protons. So going left to right the strength of force is increased which leads to squeezing of atoms. So, the radius is decreased with moving left to right in periodic table.
In other words, While we move left to right in the periodic table the neucleous charge is increased which increase electron attractions and leads to decreasing atomic radius.
Along the group
While moving up to down in the group the number of orbits is increased which leads to increase the atomic radius.
Atomic radius decreased
![]()
![]()
Atomic radius
increased
Fig-5
Exceptions
- Till now we learn that by moving left to right atomic radius decreased but in case of H2 and He the atomic radius of H2 is less than He. This is because of type of atomic radius we used. For He we use Van-dar wall atomic radius because He is an inert gas and for H2 covalent atomic radius is used. We learnt that Van-dar wall atomic radius is greater than covalent radius. So, He atomic radius is greater than H2. Same behavior of inert gases in all the period can be seen.
- In d block elements atomic radius first increase than remain constant and then increase again. This is because of the non uniform numbers of electrons in outer most shell. With increase in number of electron leads to the repulsion between them which results expansion of the size of atom.
Recommended Articles:
Asteroid and Comet Difference
Astigmatism Eye Defects: Causes, Types And Correction
Atmosphere: Definition, Layers and Significance
Atmospheric Pressure and Gauge Pressure
Read all about Atomic Number Mass Number
Basically, an atomic radius is the distance between electrons present at outermost shell to the nucleus. Heisenberg’s uncertainty principle explains about the position of electrons in the atom. It states that “it is impossible to measure or calculate exactly, both the position and the momentum of an object. This principle is based on the wave-particle duality of matter”. CH4 molecules atomic radius is determined by the covalent bond length because the electro negativity of carbon and hydrogen is same. The molecules interact to each other to form a material. This force of attraction is also called Van-dar wall force. Thus two molecules are bonded together with intermolecular attraction not by any of the bond are considered in this case. Due to this force a minimum distance can be measured from the center of two atoms which is called van-der wall atomic radius. Vegetables Names FAQs
Define atomic radius.
Explain Heisenberg’s uncertainty principle.
In a CH4 molecule the radius of carbon is 70 pm and the bond length of CH4 is 112 pm. Find out the bond length of hydrogen.
Explain van-der wall atomic radius in brief.