Introduction
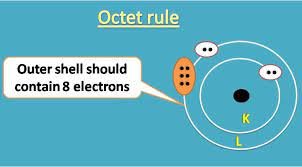
The octet rule is an essential concept in chemistry that explains the stability of atoms. This rule states that atoms have a tendency to lose or gain electrons to acquire a stable configuration of eight electrons in their outermost shell.
This process results in the formation of chemical bonds between atoms. The octet rule is significant in understanding why certain elements form particular types of bonds, such as ionic or covalent bonds. While the octet rule is generally true for many elements, there are exceptions to this rule.
Let’s delve into the octet rule and its significance in determining the stability of an atom.
What is the Octet Rule?
The octet rule is a chemical principle that states that atoms tend to gain or lose electrons to acquire a stable configuration of eight electrons in their outermost shell. This configuration is also known as the octet configuration or the noble gas configuration. The noble gases, such as helium, neon, and argon, have completely filled outermost shells and are chemically stable.
Atoms with less than eight electrons in their outermost shell tend to be reactive and can form chemical bonds to acquire the octet configuration. For example, a sodium atom has one electron in its outermost shell, which makes it highly reactive. Sodium can lose this electron to form a positively charged ion, Na+. By doing so, it attains the electron configuration of neon, which has a completely filled outermost shell.
On the other hand, atoms with more than eight electrons in their outermost shell can also be chemically unstable. These atoms can lose or gain electrons to achieve a stable octet configuration. For example, a chlorine atom has seven electrons in its outermost shell. By gaining one electron, it attains the electron configuration of argon and becomes a negatively charged ion, Cl–.
Why is the Octet Rule Important?
The octet rule is an essential concept in chemistry as it explains the behaviour of atoms in chemical reactions. Atoms tend to gain, lose, or share electrons to achieve a stable electron configuration. This process of gaining or losing electrons results in the formation of chemical bonds between atoms. The octet rule helps us understand why certain elements form particular types of bonds.
For example, ionic bonds form between atoms that transfer electrons to achieve a stable octet configuration. In an ionic bond, one atom loses electrons while another atom gains electrons. This results in the formation of positively and negatively charged ions that are attracted to each other. For example, the compound sodium chloride (NaCl) is formed when sodium donates one electron to chlorine to achieve a stable octet configuration.
Covalent bonds form between atoms that share electrons to achieve a stable octet configuration. In a covalent bond, both atoms share a pair of electrons to attain a stable electron configuration. For example, in the molecule methane (CH4), the carbon atom shares electrons with four hydrogen atoms to achieve a stable octet configuration.
Exceptions to the Octet Rule
While the octet rule holds true for many elements, there are some exceptions to this rule. Some elements, such as hydrogen and helium, have only two electrons in their outermost shell and can achieve a stable electron configuration with just two electrons. For example, the helium atom has two electrons in its outermost shell and is already in the stable configuration of a noble gas.
Another exception to the octet rule is found in molecules with an odd number of electrons. In such cases, it is impossible to achieve a stable octet configuration as the total number of electrons cannot be divided equally among the atoms. For example, in the nitrogen molecule (N2), each nitrogen atom has five electrons in its outermost shell. When they share electrons to form a covalent bond, each nitrogen atom has access to seven electrons, which is an unstable configuration. However, this configuration is still relatively stable due to the high bond energy between the two nitrogen atoms.
Frequently Asked Questions (FAQs)
Q1: What is the octet rule?
Ans: The octet rule is a chemical principle that states that atoms tend to gain, lose or share electrons to achieve a stable configuration of eight electrons in their outermost shell. This configuration is also known as the octet configuration or the noble gas configuration.
Q2: Why is the octet rule important in chemistry?
Ans: The octet rule is important in chemistry as it explains the behaviour of atoms in chemical reactions. Atoms tend to gain, lose or share electrons to achieve a stable electron configuration, resulting in the formation of chemical bonds between atoms. The octet rule helps us to understand why certain elements form particular types of bonds.
Q3: Are there any exceptions to the octet rule?
Ans: Yes, there are exceptions to the octet rule. Some elements, such as hydrogen and helium, have only two electrons in their outermost shell and can achieve a stable electron configuration with just two electrons. Additionally, molecules with an odd number of electrons cannot achieve a stable octet configuration, but they can still be relatively stable due to the high bond energy between the atoms.
Q4: How does the octet rule explain the formation of chemical bonds?
Ans: Atoms tend to gain, lose or share electrons to achieve a stable electron configuration, which results in the formation of chemical bonds. Ionic bonds form between atoms that transfer electrons to achieve a stable octet configuration, while covalent bonds form between atoms that share electrons to achieve a stable octet configuration. The octet rule helps us to predict the stability of molecules and understand the behaviour of atoms in chemical reactions.